Our Health Library information does not replace the advice of a doctor. Please be advised that this information is made available to assist our patients to learn more about their health. Our providers may not see and/or treat all topics found herein. This information is produced and provided by the National Cancer Institute (NCI). The information in this topic may have changed since it was written. For the most current information, contact the National Cancer Institute via the Internet web site at http://cancer.gov or call 1-800-4-CANCER. Carcinomas of the vagina are uncommon tumors comprising about 2% of the cancers that arise in the female genital system.[1] Squamous cell carcinoma (SCC) accounts for approximately 80% to 90% of vaginal cancer cases and adenocarcinoma accounts for 5% to 10% of vaginal cancer cases.[1] Rarely, melanomas (often nonpigmented), sarcomas, small-cell carcinomas, lymphomas, or carcinoid tumors have been described as primary vaginal cancers. The natural history, prognosis, and treatment of other primary vaginal cancers are different and are not covered in this summary. Distant hematogenous metastases occur most commonly in the lungs, and, less frequently, in the liver, bone, or other sites.[1] The American Joint Committee on Cancer staging system classifies tumors in the vagina that involve the cervix of women with an intact uterus as cervical cancers.[2] Therefore, tumors that originated in the apical vagina but extend to the cervix are classified as cervical cancers. For more information, see Cervical Cancer Treatment. Incidence and Mortality Estimated new cases and deaths from vaginal and other female genital cancer in the United States in 2025:[3] Anatomy Risk Factors Increasing age is the most important risk factor for most cancers. Other risk factors for vaginal cancer include: Vaginal adenosis is most commonly found in young women who had in utero exposure to DES and may coexist with a clear cell adenocarcinoma, although it rarely progresses to adenocarcinoma. Adenosis is replaced by squamous metaplasia, which occurs naturally, and requires follow-up but not removal. Clinical Features Although early vaginal cancer may not cause noticeable signs or symptoms, possible signs and symptoms of vaginal cancer include: Diagnostic Evaluation The following procedures may be used to diagnose vaginal cancer: Prognostic Factors Prognosis depends primarily on the stage of disease, but survival is reduced among women with the following features: In addition, the length of vaginal wall involvement has been found to be associated with survival and stage of disease in patients with vaginal SCC. Follow-Up After Treatment Similar to other gynecologic malignancies, the evidence to support surveillance after initial management of vaginal cancer is weak because of a lack of randomized or prospective clinical studies.[9] There is no reliable evidence that routine cytological or imaging procedures in patients improves health outcomes beyond what is achieved by careful physical examination and assessment of new symptoms. Therefore, outside the investigational setting, imaging procedures may be reserved for patients in whom physical examination or symptoms raise clinical suspicion of a recurrence or progression. References: FIGO Staging System The Fédération Internationale de Gynécologie et d'Obstétrique (FIGO) and the American Joint Committee on Cancer (AJCC) have designated staging to define vaginal cancer. The FIGO system is the most commonly used staging system for vaginal cancer.[1,2,3] In addition, the FIGO staging system incorporates a modified World Health Organization prognostic scoring system. The scores from the eight risk factors are summed and incorporated into the FIGO stage, separated by a colon (e.g., stage II:4, stage IV:9, etc.). Unfortunately, a variety of risk-scoring systems have been published, making comparisons of results difficult. References: Because vaginal cancer is rare, studies are limited to retrospective case series, usually from single-referral institutions.[Level of evidence C2] During the long span of time covered by these case series, available staging tests and radiation techniques often changed, including the shift to high-energy accelerators and conformal and intensity-modulated radiation therapy.[1,2] Comparing treatment approaches is further complicated by the frequent failure of investigators to provide precise staging criteria (particularly for stage I vs. stage II disease) or criteria for the choice of treatment modality. This has led to a broad range of reported disease control and survival rates for any given stage and treatment modality.[3] The following factors should be considered when planning treatment for vaginal cancer: Radiation-induced damage to nearby organs may include:[1,2] Management of the extremely rare vaginal clear cell carcinoma is similar to the management of squamous cell carcinoma. However, techniques that preserve vaginal and ovarian function should be strongly considered during treatment planning, given the young age of the patients at diagnosis.[4] For patients with early-stage vaginal carcinoma, radiation therapy, surgery, or a combination of these treatments are standard. Data from randomized trials are lacking, and the choice of therapy is generally determined by institutional experience and the factors listed above.[3] For patients with stages III and IVa disease, radiation therapy is standard and includes external-beam radiation therapy (EBRT), alone or with brachytherapy. Regional lymph nodes are included in the radiation portal. When used alone, EBRT involves a tumor dose of 65 Gy to 70 Gy, using shrinking fields, delivered within 6 to 7 weeks. Intracavitary brachytherapy provides insufficient dose penetration for locally advanced tumors, so interstitial brachytherapy is used if brachytherapy is given.[3,5] For patients with stage IVb or recurrent disease that cannot be managed with local treatments, current therapy is inadequate. No established anticancer drugs have demonstrated proven clinical benefit, although patients are often treated with regimens used to treat cervical cancer. Concurrent chemotherapy, using fluorouracil or cisplatin-based therapy, and radiation are sometimes advocated, based solely on extrapolation from cervical cancer management strategies.[6,7,8] Evidence is limited to small case series and the incremental impact on survival and local control is not well defined.[Level of evidence C3] Local control is a problem with bulky tumors. Some investigators have also used concurrent chemotherapy with agents such as cisplatin, bleomycin, mitomycin, floxuridine, and vincristine without improved outcomes.[1] It is an extrapolation from treatment approaches used in cervical cancer, based on shared etiologic and risk factors. Because vaginal cancer is rare, these patients are candidates for clinical trials of anticancer drugs and/or radiosensitizers to attempt to improve survival or local control. Discussion of clinical trials should be considered with eligible patients. Information about ongoing clinical trials is available from the NCI website. Fluorouracil Dosing The DPYD gene encodes an enzyme that catabolizes pyrimidines and fluoropyrimidines, like capecitabine and fluorouracil. An estimated 1% to 2% of the population has germline pathogenic variants in DPYD, which lead to reduced DPD protein function and an accumulation of pyrimidines and fluoropyrimidines in the body.[9,10] Patients with the DPYD*2A variant who receive fluoropyrimidines may experience severe, life-threatening toxicities that are sometimes fatal. Many other DPYD variants have been identified, with a range of clinical effects.[9,10,11] Fluoropyrimidine avoidance or a dose reduction of 50% may be recommended based on the patient's DPYD genotype and number of functioning DPYD alleles.[12,13,14]DPYD genetic testing costs less than $200, but insurance coverage varies due to a lack of national guidelines.[15] In addition, testing may delay therapy by 2 weeks, which would not be advisable in urgent situations. This controversial issue requires further evaluation.[16] References: Vaginal intraepithelial neoplasia (VaIN), the presence of noninvasive squamous cell atypia, is classified by the degree of involvement of the epithelium, as follows: VaIN is associated with a high rate of human papillomavirus (HPV) infection and is thought to have an etiology that is similar to that of cervical intraepithelial neoplasia (CIN).[1,2,3] The cervix and vulva are carefully evaluated because vaginal carcinoma in situ is associated with other genital neoplasia, and in some cases, may be an extension of CIN. Vaginal carcinoma in situ is often multifocal and commonly occurs in the vaginal vault. For more information, see Cervical Cancer Treatment. The extent and type of surgical treatment needed is dependent upon anatomical location, evidence of multifocality, general patient comorbidities, and other specific factors (e.g., anatomical distortion of vaginal vault from prior hysterectomy).[4] Treatment Options for VaIN The following treatments have not been directly compared in randomized trials, so their relative efficacy is uncertain.[Level of evidence C3] Treatment options for VaIN include: Women with VaIN 1 can usually be observed carefully without ablative or surgical treatment because the lesions often regress spontaneously. VaIN 2, the intermediate grade, is managed by careful observation or initial treatment. Although the natural history of VaIN is not precisely known because of its rarity, patients with VaIN 3 are presumed to be at substantial risk of progression to invasive cancer and are treated immediately. Lesions with hyperkeratosis respond better to excision or laser vaporization than to 5-FU.[4] Current Clinical Trials Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available. References: The treatment options for stage I vaginal cancer have not been directly compared in randomized trials.[Level of evidence C2] Because of differences in patient selection, expertise in treating local disease, and staging criteria, it is difficult to determine whether there are differences in disease control rates. Treatment Options for Stage I Squamous Cell Carcinoma (SCC) of the Vagina Treatment options for stage I SCC of the vagina superficial lesions less than 0.5 cm thick include: Treatment options for stage I SCC of the vagina lesions more than 0.5 cm thick include: Treatment Options for Stage I Adenocarcinoma of the Vagina Treatment options for stage I adenocarcinoma of the vagina include: Current Clinical Trials Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available. References: The treatment options for stages II, III, and IVa vaginal cancer have not been directly compared in randomized trials.[Level of evidence C2] As a result of differences in patient selection, expertise in treating local disease, and staging criteria, it is difficult to determine whether there are differences in disease control rates. Radiation therapy is the most common treatment for patients with stages II, III, and IVa vaginal cancer. Treatment Options for Stages II, III, and IVa Squamous Cell Carcinoma (SCC) and Adenocarcinoma of the Vagina Treatment options for stage II SCC and adenocarcinoma of the vagina, stage III SCC and adenocarcinoma of the vagina, and stage IVa SCC and adenocarcinoma of the vagina include: Current Clinical Trials Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available. References: Treatment Options for Stage IVb Squamous Cell Carcinoma (SCC) and Adenocarcinoma of the Vagina Treatment options for stage IVb SCC and adenocarcinoma of the vagina include: For patients with stage IVb disease, current therapy is inadequate. No established anticancer drugs have demonstrated clinical benefit, although patients are often treated with regimens used to treat cervical cancer. Concurrent chemotherapy using fluorouracil or cisplatin-based therapy and radiation therapy is sometimes advocated on the basis of results extrapolated from cervical cancer management strategies.[1,2,3] Evidence is limited to small case series, and the incremental impact on patient survival and local disease control is not well defined.[Level of evidence C3] For more information, see Cervical Cancer Treatment. Because stage IVb vaginal cancer is rare, these patients are candidates for clinical trials to improve survival or local control. Information about ongoing clinical trials is available from the NCI website. Current Clinical Trials Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available. References: Patients with recurrent vaginal cancer have a very poor prognosis. Most recurrences occur in the first 2 years after treatment. Some patients with centrally recurrent vaginal cancers are candidates for pelvic exenteration or radiation therapy. In a large series, only 5 of 50 patients with recurrence were salvaged using surgery or radiation therapy. All five of these salvaged patients originally presented with stage I or II disease and had tumor recurrence in the central pelvis.[1] No established anticancer drugs have demonstrated clinical benefit, although patients are often treated with regimens used to treat cervical cancer. If patients are eligible, participation in clinical trials should be considered. Information about ongoing clinical trials is available from the NCI website. Current Clinical Trials Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available. References: The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above. General Information About Vaginal Cancer Updated statistics with estimated new cases and deaths for 2025 (cited American Cancer Society as reference 3). This summary is written and maintained by the PDQ Adult Treatment Editorial Board, which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® Cancer Information for Health Professionals pages. Purpose of This Summary This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the treatment of vaginal cancer. It is intended as a resource to inform and assist clinicians in the care of their patients. It does not provide formal guidelines or recommendations for making health care decisions. Reviewers and Updates This summary is reviewed regularly and updated as necessary by the PDQ Adult Treatment Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH). Board members review recently published articles each month to determine whether an article should: Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary. The lead reviewers for Vaginal Cancer Treatment are: Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's Email Us. Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries. Levels of Evidence Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Adult Treatment Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations. Permission to Use This Summary PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as "NCI's PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary]." The preferred citation for this PDQ summary is: PDQ® Adult Treatment Editorial Board. PDQ Vaginal Cancer Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/vaginal/hp/vaginal-treatment-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389242] Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online, a collection of over 2,000 scientific images. Disclaimer Based on the strength of the available evidence, treatment options may be described as either "standard" or "under clinical evaluation." These classifications should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page. Contact Us More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website's Email Us. Last Revised: 2025-04-03 This information does not replace the advice of a doctor. Ignite Healthwise, LLC disclaims any warranty or liability for your use of this information. Your use of this information means that you agree to the Terms of Use and Privacy Policy. Learn how we develop our content. Healthwise, Healthwise for every health decision, and the Healthwise logo are trademarks of Ignite Healthwise, LLC.Topic Contents
Vaginal Cancer Treatment (PDQ®): Treatment - Health Professional Information [NCI]
General Information About Vaginal Cancer
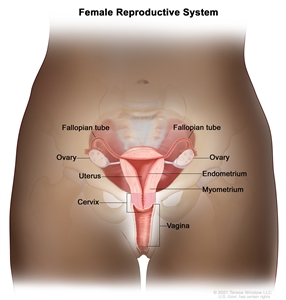
Normal female reproductive system anatomy.Stage Information for Vaginal Cancer
FIGO Nomenclature FIGO = Fédération Internationale de Gynécologie et d'Obstétrique. a Adapted from FIGO Committee on Gynecologic Oncology.[1,2] Stage I The carcinoma is limited to the vaginal wall. Stage II The carcinoma has involved the subvaginal tissue but has not extended to the pelvic wall. Stage III The carcinoma has extended to the pelvic wall. Stage IV The carcinoma has extended beyond the true pelvis or has involved the mucosa of the bladder or rectum; bullous edemas as such does not permit a case to be allotted to stage IV. IVa - Tumor invades bladder and/or rectal mucosa and/or direct extension beyond the true pelvis. IVb - Spread to distant organs. Treatment Option Overview for Vaginal Cancer
Stage (FIGO Staging System) Treatment Options FIGO = Fédération Internationale de Gynécologie et d'Obstétrique; SCC = squamous cell carcinoma; VaIN = vaginal intraepithelial neoplasia. VaIN (this stage is not recognized by FIGO) Laser therapy Wide local excision Vaginectomy Intravaginal chemotherapy Intracavitary radiation therapy Imiquimod Stage I vaginal cancer SCC Radiation therapy Surgery Adenocarcinoma Surgery Radiation therapy Combined local therapy Stages II, III, and IVa vaginal cancer (SCC and adenocarcinoma) Radiation therapy Surgery Chemoradiation Stage IVb vaginal cancer (SCC and adenocarcinoma) Radiation therapy Treatment of Vaginal Intraepithelial Neoplasia
Treatment of Stage I Vaginal Cancer
Treatment of Stages II, III, and IVa Vaginal Cancer
Treatment of Stage IVb Vaginal Cancer
Treatment of Recurrent Vaginal Cancer
Latest Updates to This Summary (04 / 03 / 2025)
About This PDQ Summary
Our Health Library information does not replace the advice of a doctor. Please be advised that this information is made available to assist our patients to learn more about their health. Our providers may not see and/or treat all topics found herein.Vaginal Cancer Treatment (PDQ®): Treatment - Health Professional Information [NCI]



