Our Health Library information does not replace the advice of a doctor. Please be advised that this information is made available to assist our patients to learn more about their health. Our providers may not see and/or treat all topics found herein. This information is produced and provided by the National Cancer Institute (NCI). The information in this topic may have changed since it was written. For the most current information, contact the National Cancer Institute via the Internet web site at http://cancer.gov or call 1-800-4-CANCER. There are three main types of skin cancer: BCC and SCC are the most common forms of skin cancer and together are referred to as nonmelanoma skin cancers. This summary addresses the treatment of BCC and SCC of the skin and the related noninvasive lesion actinic keratosis. For more information about the treatment of melanoma, see Melanoma Treatment. Incidence and Mortality Nonmelanoma skin cancer is the most common cancer in the United States. BCC is the more common type, accounting for about three-quarters of nonmelanoma skin cancers.[1] The incidence of nonmelanoma skin cancer appears to be increasing in some,[2] but not all,[3] areas of the United States. Overall U.S. incidence rates have likely been increasing for a number of years.[4] At least some of this increase may be attributable to increasing skin cancer awareness and the resulting examination and biopsy of skin lesions. The total number and incidence rate of nonmelanoma skin cancers cannot be estimated precisely because reporting to cancer registries is not required. However, based on extrapolation of Medicare fee-for-service data to the U.S. population, it has been estimated that the total number of people treated for nonmelanoma skin cancers in 2012 was about 3.3 million.[5,6] That number exceeds all other annual new cases of cancer estimated by the American Cancer Society, which total about 2 million.[6] Although nonmelanoma skin cancer is the most common of all malignancies, it accounts for less than 0.1% of patient deaths caused by cancer. Anatomy Risk Factors Risk factors for nonmelanoma skin cancer include the following: Types of Skin Cancer This evidence-based summary covers basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) of the skin and the related noninvasive lesion actinic keratosis (viewed by some pathologists as a variant of in situ SCC).[1] BCC and SCC are both of epithelial origin. Although BCC and SCC are by far the most frequent types of nonmelanoma skin cancers, approximately 82 types of skin malignancies, with a wide range of clinical behaviors, fall into the category of nonmelanoma skin cancer.[10] Other types of malignant disease of the skin include the following: For more information, see Melanoma Treatment, Merkel Cell Carcinoma Treatment, Mycosis Fungoides and Other Cutaneous T-Cell Lymphomas Treatment, and Kaposi Sarcoma Treatment. Basal cell carcinoma BCC is at least three times more common than SCC in nonimmunocompromised patients. It usually occurs on sun-exposed areas of skin, with the nose being the most common site. Although there are many different clinical presentations for BCC, the most characteristic type is the asymptomatic nodular or nodular ulcerative lesion that is elevated from the surrounding skin, has a pearly quality, and contains telangiectatic vessels. BCCs are composed of nonkeratinizing cells derived from the basal cell layer of the epidermis. They are slow growing and rarely metastasize. BCC has a tendency to be locally destructive and can result in serious deforming damage if left untreated or if local recurrences cannot be completely excised. High-risk areas for tumor recurrence after initial treatment include the central face (e.g., periorbital region, eyelids, nasolabial fold, or nose-cheek angle), postauricular region, pinna, ear canal, forehead, and scalp.[11] Morpheaform type is a specific BCC subtype. This subtype typically appears as a scar-like, firm plaque. Because of indistinct clinical tumor margins, morpheaform type is difficult to treat adequately with traditional treatments.[12] BCCs often have a characteristic mutation in the PTCH1 tumor suppressor gene, although the mechanism of carcinogenesis is not clear.[1] Squamous cell carcinoma People with chronic sun damage, history of sunburns, arsenic exposure, chronic cutaneous inflammation (as seen in long-standing skin ulcers), and previous radiation therapy are predisposed to the development of SCC. SCCs tend to occur on sun-exposed portions of the skin, such as the ears, lower lip, and dorsa of the hands. SCCs that develop from actinic keratosis on sun-exposed skin are less likely to metastasize and have a better prognosis than those that develop de novo, or on non–sun-exposed skin.[12] SCCs are composed of keratinizing cells. These tumors are more aggressive than BCCs and have a range of growth, invasive, and metastatic potential. Prognosis is associated with the degree of differentiation, and tumor grade is reported as part of the staging system.[10] A four-grade system (G1–G4) is most common, but two- and three-grade systems may also be used. Mutations in the PTCH1 tumor suppressor gene have been reported in SCCs removed from patients with a prior history of multiple BCCs.[13] SCC in situ (also called Bowen disease) is a noninvasive lesion. Distinguishing SCC in situ pathologically from a benign inflammatory process may be difficult.[1] The risk of development into invasive SCC is low, reportedly in the range of 3% to 4%.[14] Actinic keratosis Actinic keratoses are potential precursors of SCC, but the rate of progression is extremely low, and most do not become SCCs. These typically red, scaly patches usually arise on areas of chronically sun-exposed skin and are likely to be found on the face and dorsal aspects of the hand. Diagnostic and Staging Evaluation BCC and SCC are usually diagnosed on the basis of routine histopathology obtained from a shave, punch, incisional, or excisional biopsy.[1] Other tests and procedures that may be used to diagnose and stage BCC and SCC of the skin include the following: Ophthalmic examination or evaluation is performed to diagnose and stage eyelid carcinoma. References: There are separate staging systems in the 8th edition of the American Joint Committee on Cancer's (AJCC's) AJCC Cancer Staging Manual for carcinoma of the eyelid and for cutaneous carcinoma of the head and neck. The cutaneous carcinoma staging system addresses cutaneous squamous cell carcinoma (SCC) and cutaneous basal cell carcinoma (BCC).[1,2] The staging system for carcinomas of the eyelid addresses carcinomas of all histologies. Regional lymph nodes should be routinely examined in all cases of SCC, especially for the following: BCC rarely metastasizes, so a metastatic workup is usually not necessary. There are several factors that correlate with poor prognosis for recurrence and metastasis. They apply primarily to patients with SCC and an aggressive subset of nonmelanoma skin carcinoma, but rarely to patients with BCC, and include the following:[1] Even with relatively small tumor sizes, SCCs that occur in immunosuppressed patients tend to behave more aggressively than do SCCs in nonimmunosuppressed patients. Although immunosuppression is not a formal part of the AJCC staging system, it is recommended that centers prospectively studying SCC record the presence and type of immunosuppression. Staging for Cutaneous Carcinoma of the Head and Neck (Excluding Carcinomas of the Eyelid) The AJCC has designated staging by TNM (tumor, node, metastasis) classification for cutaneous carcinoma of the head and neck, excluding carcinomas of the eyelid.[1] Staging for Carcinomas of the Eyelid The AJCC has designated staging by TNM classification.[1] The TNM classification is used to stage all cell types of eyelid carcinomas, except melanoma. References: Treatments for squamous cell carcinoma and basal cell carcinoma of the skin are described in Table 10. There is a wide range of approaches for treating basal cell carcinoma (BCC) of the skin, including excision, radiation therapy, cryosurgery, electrodesiccation and curettage, photodynamic or laser-beam light exposure, and topical therapies. Each of these approaches is useful in specific clinical situations. Depending on case selection, these approaches have recurrence-free rates ranging from 85% to 95%.[1,2,3,4,5,6,7,8,9] A systematic review of 27 randomized controlled trials comparing various treatments for BCC has been published.[10] Eighteen of the studies were published in full, and nine were published in abstract form only. Only 19 of the 27 trials were analyzed by intention-to-treat criteria. Because the case fatality rate of BCC is so low, the primary end point of most trials is complete response and/or recurrence rate after treatment. Most of the identified studies were not of high quality and had short follow-up periods, which will lead to overestimates of tumor control; only one study had a follow-up period of as long as 4 years. A literature review of recurrence rates in case series with long-term follow-up after treatment of BCCs indicated that only 50% of recurrences occurred within the first 2 years, 66% after 3 years, and 18% after 5 years.[11] A common finding was that the 10-year recurrence rates were about double the 2-year recurrence rates. Treatment of Basal Cell Carcinoma of the Skin (Localized Disease) Treatment options for BCC of the skin (localized disease) include the following: Surgical excision with margin evaluation A traditional surgical treatment, surgical excision with margin evaluation usually relies on surgical margins ranging from 3 mm to 10 mm, depending on the diameter of the tumor. Re-excision may be required if the surgical margin is found to be inadequate on permanent sectioning. In one trial, 35 of 199 primary BCCs (18%) were incompletely excised by the initial surgery and underwent a re-excision.[12] In addition, many laboratories examine only a small fraction of the total tumor margin pathologically. Therefore, the declaration of tumor-free margins can be subject to sampling error.[13] In randomized trials, excision has been compared with radiation therapy, Mohs micrographic surgery, photodynamic therapy (PDT), and cryosurgery. Evidence (surgical excision with margin evaluation): Mohs micrographic surgery Mohs micrographic surgery is a form of tumor excision that involves progressive radial sectioning and real-time examination of the resection margins until adequate uninvolved margins have been achieved, avoiding wider margins than needed. It is a specialized technique used to achieve the narrowest margins necessary to avoid tumor recurrence while maximally preserving cosmesis. The tumor is microscopically delineated, with serial radial resection, until it is completely removed as assessed with real-time frozen sections. Noncontrolled case series suggested that the disease control rates were superior to other treatment methods for BCC.[18,19,20] However, as noted in the Surgical excision with margin evaluation section, the disease control rate was not clearly better when it was directly compared with the disease control rate for surgical excision of facial BCCs in a randomized trial of primary BCCs.[12] This surgery is best suited to the management of tumors that have recurred after initial incision or of tumors in cosmetically sensitive areas (e.g., eyelid periorbital area, nasolabial fold, nose-cheek angle, posterior cheek sulcus, pinna, ear canal, forehead, scalp, fingers, and genitalia).[19,21] It is also used to treat tumors with poorly defined clinical borders. Radiation therapy Radiation therapy is particularly useful in the management of patients with primary lesions that would otherwise require difficult or extensive surgery (e.g., lesions on the nose or ears).[22] Radiation therapy eliminates the need for skin grafting when surgery would result in an extensive defect. Cosmetic results are generally good, with a small amount of hypopigmentation or telangiectasia in the treatment port. Radiation therapy can also be used for lesions that recur after a primary surgical approach.[23] Radiation therapy is avoided in patients with conditions that predispose them to radiation-induced cancers, such as xeroderma pigmentosum or basal cell nevus syndrome. Evidence (radiation therapy): Curettage and electrodesiccation Curettage and electrodesiccation is a widely employed method for removing primary BCCs, especially superficial lesions of the neck, trunk, and extremities that are considered to be at low risk of recurrence. A sharp curette is used to scrape the tumor down to its base, followed by electrodesiccation of the lesion base. Although curettage and electrodesiccation is a quick method for destroying the tumor, the adequacy of treatment cannot be assessed immediately because the surgeon cannot visually detect the depth of microscopic tumor invasion. This procedure is also sometimes called electrosurgery. Evidence (curettage and electrodesiccation): Cryosurgery Cryosurgery may be considered for patients with small, clinically well-defined primary tumors.[26,27,28] It is infrequently used for the management of BCC, but cryosurgery may be useful for patients with medical conditions that preclude other types of surgery.[8,29,30,31,32,33,34,35] Contraindications for cryosurgery include the following: Caution should also be used before treating nodular ulcerative neoplasia more than 3 cm in diameter, carcinomas fixed to the underlying bone or cartilage, tumors situated on the lateral margins of the fingers and at the ulnar fossa of the elbow, or recurrent carcinomas following surgical excision. Permanent pigment loss at the treatment site is unavoidable, so the treatment is not well suited to patients with dark skin. Edema is common after treatment, especially around the periorbital region, temple, and forehead. Treated tumors usually exude necrotic material, after which an eschar forms and persists for about 4 weeks. Atrophy and hypertrophic scarring have been reported, as have instances of motor and sensory neuropathy. Evidence (cryosurgery): Photodynamic therapy PDT with photosensitizers is used in the management of a wide spectrum of superficial epithelial tumors.[39] A topical photosensitizing agent such as 5-aminolevulinic acid or methyl aminolevulinate is applied to the tumor, followed by exposure to a specific wavelength of light (laser or broad band), depending on the absorption characteristics of the photosensitizer. In the case of multiple BCCs, the use of short-acting systemic (intravenous) photosensitizers such as verteporfin has been investigated.[40] Upon light activation, the photosensitizer reacts with oxygen in the tissue to form singlet oxygen species, resulting in local cell destruction. Evidence (PDT): Topical fluorouracil (5-FU) Topical 5-FU, as a 5% cream, may be useful in specific limited circumstances. The U.S. Food and Drug Administration (FDA) approved this treatment for superficial BCCs in patients for whom conventional methods are impractical, such as individuals with multiple lesions or difficult treatment sites. Safety and efficacy in other indications have not been established.[41,42][Level of evidence C3] Given the superficial nature of the effects of topical 5-FU, nonvisible dermal involvement may persist, giving a false impression of treatment success. In addition, the brisk accompanying inflammatory reaction may cause substantial skin toxicity and discomfort in a large proportion of patients. Imiquimod topical therapy Imiquimod is an agonist for the toll-like receptor 7 and/or 8, inducing a helper T-cell cytokine cascade and interferon production. It purportedly acts as an immunomodulator. Although the FDA approved imiquimod for treatment of superficial BCCs, some investigators in the field do not recommend it for initial monotherapy for BCC. Some reserve its use for patients with small lesions in low-risk sites who cannot undergo treatment with more established therapies.[42] Imiquimod is available as a 5% cream and is used in schedules ranging from twice weekly to twice daily over 5 to 15 weeks. Most of the experience is limited to case series of BCCs that are smaller than 2 cm2 in area and that are not in high-risk locations (e.g., within 1 cm of the hairline, eyes, nose, mouth, or ear; or in the anogenital, hand, or foot regions).[42] Follow-up times have also been generally short. Reported CR rates vary widely, from about 40% to 100%.[42][Level of evidence C3] There have been a number of randomized trials of imiquimod.[43,44,45,46,47,48] However, the designs of all of them make interpretation of long-term efficacy impossible. Most were industry-sponsored dose-finding studies, with small numbers of patients on any given regimen; and patients were only monitored for 6 to 12 weeks, with excision at that time to determine histological response.[42][Level of evidence B3] Carbon dioxide laser The carbon dioxide laser is used very infrequently in the management of BCC because of the difficulty in controlling tumor margins.[49] Few clinicians have extensive experience with the technique for BCC treatment. There are no randomized trials comparing it with other modalities. Treatment of Metastatic Basal Cell Carcinoma (or Locally Advanced Disease Untreatable by Local Modalities) Treatment options for metastatic BCC of the skin (or locally advanced disease untreatable by local modalities) include the following: Hedgehog pathway inhibitors BCCs frequently exhibit constitutive activation of the Hedgehog/PTCH1 signaling pathway. Vismodegib and sonidegib, two inhibitors of Smoothened, a transmembrane protein involved in the Hedgehog pathway, are approved for the treatment of adults with metastatic BCC, patients with locally advanced BCC that has recurred after surgery, and patients who are not candidates for surgery or radiation therapy. Evidence (vismodegib): Evidence (sonidegib): Chemotherapy No standard chemotherapy regimens exist, and there are only anecdotal reports in the literature.[52] Because there is no curative therapy for metastatic BCC of the skin, clinical trials are appropriate. Information about ongoing clinical trials is available from the NCI website. Treatment of Recurrent Nonmetastatic Basal Cell Carcinoma of the Skin After treatment of BCC, patients are monitored clinically and examined regularly. Most recurrences occur within 5 years, but about 18% of recurrences are diagnosed beyond that point.[11] Patients who develop a primary BCCs are also at increased risk of subsequent primary skin cancers because their sun-damaged skin is susceptible to additional cancers.[53,54,55] This effect is sometimes termed field carcinogenesis. Age at diagnosis of the first BCC (<65 years), red hair, and initial BCC on the upper extremities appear to be associated with a higher risk of subsequent new BCCs.[56] Treatment options for recurrent nonmetastatic BCC of the skin include the following: Mohs micrographic surgery is commonly used for local recurrences of BCC. Evidence (surgical excision vs. Mohs micrographic surgery): Current Clinical Trials Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available. References: Localized squamous cell carcinoma (SCC) of the skin is a highly curable disease.[1] There are a variety of treatment approaches to localized SCC, including excision, radiation therapy, cryosurgery, and electrodesiccation and curettage. There is little to no good-quality evidence that allows direct comparison of outcomes for patients with sporadic, clinically localized SCCs treated with local therapies. A systematic literature review found only one randomized controlled trial in the management of such patients, and that trial compared adjuvant therapy with observation after initial local therapy rather than different local therapies.[2] In that small single-center trial, 66 patients with high-risk, clinically localized SCC were randomly assigned, after surgical excision of the primary tumor (with or without radiation, depending on clinical judgment), to either receive combined isotretinoin (1 mg/kg orally per day) plus interferon alpha (3 × 106 U subcutaneously 3 times/week) for 6 months or undergo observation.[3] In the 65 evaluable patients after a median follow-up of 21.5 months, there was no difference in the combined (primary) end point of SCC recurrence or second primary tumor (45% vs. 38%; hazard ratio, 1.13; 95% confidence interval [CI], 0.53–2.41), or in either of the individual components of the primary end point.[3][Level of evidence B1] Cemiplimab and pembrolizumab, programmed death receptor-1 (PD-1) inhibitors, are the only systemic therapies for the treatment of locally advanced and metastatic cutaneous SCC. The U.S. Food and Drug Administration (FDA) approved cemiplimab and pembrolizumab on the basis of objective response rates (RRs) from early-phase trials.[4,5,6][Level of evidence C3] Toxicities associated with checkpoint inhibitors were seen, including death. Clinical trials are recommended to further identify optimal treatment. Ongoing trials include PD-1 inhibitors in the neoadjuvant, adjuvant, and advanced/metastatic settings, as monotherapy and in combinations. Treatment of Squamous Cell Carcinoma of the Skin (Localized Disease) Treatment options for SCC of the skin (localized disease) include the following: Surgical excision with margin evaluation Excision is probably the most common therapy for SCC.[7] This traditional surgical treatment usually relies on surgical margins ranging from 4 mm to 10 mm, depending on the diameter of the tumor and degree of differentiation. In a prospective case series of 141 SCCs, a 4-mm margin was adequate to encompass all subclinical microscopic tumor extension in more than 95% of well-differentiated tumors up to 19 mm in diameter. Wider margins of 6 mm to 10 mm were needed for larger or less-differentiated tumors and tumors in high-risk locations (e.g., scalp, ears, eyelids, nose, and lips).[8] Re-excision may be required if the surgical margin is inadequate on permanent sectioning. Mohs micrographic surgery Mohs micrographic surgery is a form of tumor excision that involves progressive radial sectioning and real-time examination of the resection margins until adequate uninvolved margins have been achieved, avoiding wider margins than needed. It is a specialized technique used to achieve the narrowest margins necessary to avoid tumor recurrence while maximally preserving cosmesis. The tumor is microscopically delineated, with serial radial resection, until it is completely removed as assessed with real-time frozen sections. However, because the technique removes tumor growing in contiguity and may miss noncontiguous in-transit cutaneous micrometastases, some practitioners remove an additional margin of skin in high-risk lesions, even after the Mohs surgical procedure confirms uninvolved margins.[7][Level of evidence C3] In case series, Mohs surgery has been associated with a lower local recurrence rate than the other local modalities,[9] but there are no randomized trials allowing direct comparison.[2] This surgery is best suited to the management of tumors in cosmetically sensitive areas (e.g., eyelid periorbital area, nasolabial fold, nose-cheek angle, posterior cheek sulcus, pinna, ear canal, forehead, scalp, fingers, and genitalia) or for tumors that have recurred after initial excision.[10,11] Mohs micrographic surgery is also used to treat high-risk tumors with poorly defined clinical borders or with perineural invasion. Radiation therapy Radiation therapy is a logical treatment choice, particularly for patients with primary lesions requiring difficult or extensive surgery (e.g., lesions on the nose, lips, or ears).[7,12] Radiation therapy eliminates the need for skin grafting in cases where surgery would result in an extensive defect. Cosmetic results are generally good, with a small amount of hypopigmentation or telangiectasia in the treatment port. Radiation therapy can also be used for lesions that recur after a primary surgical approach.[13] Radiation therapy is avoided in patients with conditions that predispose them to radiation-induced cancers, such as xeroderma pigmentosum or basal cell nevus syndrome. Although radiation therapy, with or without excision of the primary tumor, is used for histologically proven clinical lymph node metastases and has been associated with favorable disease-free survival rates, the retrospective nature of these case series makes it difficult to know the impact of nodal radiation on survival.[14,15][Level of evidence C2] Curettage and electrodesiccation Curettage and electrodesiccation is used to treat SCC of the skin. A sharp curette is used to scrape the tumor down to its base, followed by electrodesiccation of the lesion base. Although curettage and electrodesiccation is a quick method for destroying the tumor, the adequacy of treatment cannot be assessed immediately because the surgeon cannot visually detect the depth of microscopic tumor invasion. Its use is limited to small (<1 cm), well-defined, and well-differentiated tumors.[7][Level of evidence C2] This procedure is also sometimes called electrosurgery. Cryosurgery Cryosurgery may be considered for patients with small, clinically well-defined primary tumors. It may be useful for patients with medical conditions that preclude other types of surgery.[16,17] Contraindications for cryosurgery include the following: Caution should also be used before treating nodular ulcerative neoplasia larger than 3 cm in diameter, carcinomas fixed to the underlying bone or cartilage, tumors situated on the lateral margins of the fingers and at the ulnar fossa of the elbow, or recurrent carcinomas following surgical excision. Permanent pigment loss at the treatment site is unavoidable, so the treatment is not well suited to patients with dark skin. Edema is common after treatment, especially around the periorbital region, temple, and forehead. Treated tumors usually exude necrotic material, after which an eschar forms and persists for about 4 weeks. Atrophy and hypertrophic scarring have been reported, as have instances of motor and sensory neuropathy. Treatment of SCC in situ (Bowen disease) The management of SCC in situ (Bowen disease) is similar to that for good-risk SCC. However, because Bowen disease is noninvasive, surgical excision, including Mohs micrographic surgery, is usually not necessary. In addition, high complete response (CR) rates are achievable with photodynamic therapy (PDT). Evidence (PDT): Treatment of Metastatic Squamous Cell Carcinoma (or Advanced Disease Untreatable by Local Modalities) As is the case with basal cell carcinoma (BCC), metastatic and far-advanced SCC is unusual, and reports of systemic therapy are limited to case reports, small case series, or early-phase trials with tumor response as the end point.[Level of evidence C3] The metastatic rate for primary tumors of sun-exposed skin is 5%; for tumors of the external ear, 9%; and for tumors of the lip, 14%. Metastases occur at an even higher rate (about 38%) for primary SCCs in scar carcinomas or in nonexposed areas of skin.[9] About 69% of metastases are diagnosed within 1 year, 91% within 3 years, and 96% within 5 years. Immunotherapy (PD-1 inhibitors) Two PD-1 inhibitors, cemiplimab and pembrolizumab, have been approved by the FDA as systemic therapy for recurrent or metastatic SCC not amenable to curative surgery or radiation therapy (cemiplimab, pembrolizumab) and locally advanced SCC not amenable to curative surgery (cemiplimab). Cemiplimab The FDA approved cemiplimab for systemic therapy for metastatic or locally advanced SCC not amenable to curative surgery or radiation therapy. Approval was based on RR as assessed by an independent review committee in two open-label, multicenter, early-phase trials.[4,5][Level of evidence C3] The FDA-approved dose is a fixed-dose equivalent (350 mg as a 30-minute intravenous [IV] infusion administered every 3 weeks) of the trial dose given as 3 mg/kg IV over 30 minutes every 2 weeks. Toxicities associated with checkpoint inhibitors were seen, including death. Evidence (cemiplimab): Patients were required to have at least one measurable lesion and were excluded for an autoimmune disease that required systemic therapy within 5 years, previous checkpoint inhibitor therapy, solid organ transplant, Eastern Cooperative Oncology Group performance status below 1, and hepatitis or infection with HIV. Patients received treatment with 3 mg/kg IV every 2 weeks until progressive disease. RRs were assessed by an independent review committee using RECIST criteria for radiological scans and World Health Organization criteria for medical photography for a composite response. RRs were assessed after all patients had at least 6 months of follow-up. Pembrolizumab Pembrolizumab is approved for systemic therapy for recurrent or metastatic SCC not amenable to surgery or radiation therapy. Approval was based on RR as assessed by an independent review committee of a multicenter, multicohort, open-label phase II trial in patients with recurrent or metastatic SCC not amenable to surgery or radiation therapy.[6][Level of evidence C3] The cohort of patients with locally advanced disease is not yet reported. Patients received pembrolizumab 200 mg every 3 weeks. An alternate dosing regimen of pembrolizumab, 400 mg every 6 weeks, is approved across all adult indications on the basis of pharmacokinetic modeling and exposure-response analyses. Evidence (pembrolizumab): As treatment options and long-term outcomes are limited, clinical trials are recommended. Trial options include PD-1 inhibitors and cemiplimab in the advanced setting, as well as in the neoadjuvant and adjuvant settings; other checkpoint inhibitors; checkpoint inhibitor combinations; and combinations with epidermal growth factor receptor inhibitors. Treatment of Recurrent Nonmetastatic Squamous Cell Carcinoma of the Skin SCCs have definite metastatic potential, and patients are monitored regularly after initial treatment. Overall, local recurrence rates after treatment of primary SCCs have ranged from about 3% to 23%, depending on anatomical site.[9] About 58% of local recurrences manifest within 1 year, 83% within 3 years, and 95% within 5 years. Tumors that are 2 cm or larger in diameter, 4 mm or greater in depth, or poorly differentiated have a relatively poor prognosis [19] and even higher local recurrence and metastasis rates than those listed.[9] Reported local recurrence rates also vary by treatment modality, with the lowest rates associated with Mohs micrographic surgery. However, at least some of the variation may be the result of patient selection factors. No randomized trials directly compare the various local treatment modalities. Treatment options for recurrent nonmetastatic SCCs include the following: Recurrent nonmetastatic SCCs are considered high risk and are generally treated with excision, often using Mohs micrographic surgery. Radiation therapy is used for lesions that cannot be completely resected. As is the case with BCC, patients who develop a primary SCC are also at increased risk of subsequent primary skin cancers.[20,21] Current Clinical Trials Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available. References: Actinic keratoses commonly appear in areas of chronic sun exposure, such as the face and dorsa of the hands. Actinic cheilitis is a related condition that usually appears on the lower lips.[1] These conditions represent early epithelial transformation that may eventually evolve into invasive squamous cell carcinoma (SCC). Actinic keratoses are noninvasive lesions. The progression rate is extremely low. In a prospective study, the progression rate to SCC was less than 1 in 1,000 per year, calling into question the cost-effectiveness of treating all actinic keratoses to prevent SCC.[2] Moreover, in a population-based longitudinal study, there was a spontaneous regression rate of approximately 26% for solar keratoses within 1 year of a screening examination.[3] Therefore, studies designed to test the efficacy of any treatment for progression of actinic keratoses to SCC are impractical (or impossible). Nevertheless, a variety of treatment approaches have been reviewed.[4] Treatment options for actinic keratosis depend on whether the lesions are isolated or whether there are multiple lesions in the same field. Treatment options for actinic keratosis (not listed hierarchically) include the following: Current Clinical Trials Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available. References: The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above. General Information About Skin Cancer Updated American Cancer Society as reference 6. This summary is written and maintained by the PDQ Adult Treatment Editorial Board, which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® Cancer Information for Health Professionals pages. Purpose of This Summary This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the treatment of skin cancer. It is intended as a resource to inform and assist clinicians in the care of their patients. It does not provide formal guidelines or recommendations for making health care decisions. Reviewers and Updates This summary is reviewed regularly and updated as necessary by the PDQ Adult Treatment Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH). Board members review recently published articles each month to determine whether an article should: Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary. The lead reviewer for Skin Cancer Treatment is: Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's Email Us. Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries. Levels of Evidence Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Adult Treatment Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations. Permission to Use This Summary PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as "NCI's PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary]." The preferred citation for this PDQ summary is: PDQ® Adult Treatment Editorial Board. PDQ Skin Cancer Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/skin/hp/skin-treatment-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389366] Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online, a collection of over 2,000 scientific images. Disclaimer Based on the strength of the available evidence, treatment options may be described as either "standard" or "under clinical evaluation." These classifications should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page. Contact Us More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website's Email Us. Last Revised: 2025-03-07 This information does not replace the advice of a doctor. Ignite Healthwise, LLC disclaims any warranty or liability for your use of this information. Your use of this information means that you agree to the Terms of Use and Privacy Policy. Learn how we develop our content. Healthwise, Healthwise for every health decision, and the Healthwise logo are trademarks of Ignite Healthwise, LLC.Topic Contents
Skin Cancer Treatment (PDQ®): Treatment - Health Professional Information [NCI]
General Information About Skin Cancer
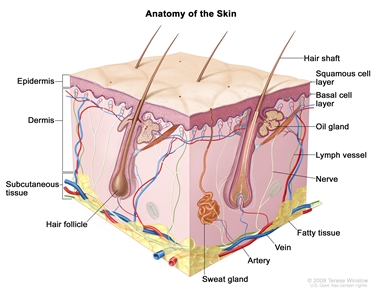
Anatomy of the skin showing the epidermis (including the squamous cell and basal cell layers), dermis, subcutaneous tissue, and other parts of the skin.Stage Information for Skin Cancer
T Category T Criteria a Reprinted with permission from AJCC: Cutaneous carcinoma of the head and neck. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 171–81. b Deep invasion is defined as invasion beyond the subcutaneous fat or >6 mm (as measured from the granular layer of adjacent normal epidermis to the base of the tumor); perineural invasion for T3 classification is defined as tumor cells within the nerve sheath of a nerve lying deeper than the dermis or measuring ≥0.1 mm in caliber, or presenting with clinical or radiographic involvement of named nerves without skull base invasion or transgression. TX Primary tumor cannot be identified. Tis Carcinomain situ. T1 Tumor ≤2 cm in greatest dimension. T2 Tumor >2 cm, but ≤4 cm in greatest dimension. T3 Tumor >4 cm in maximum dimension or minor bone erosion or perineural invasion or deep invasion.b T4 Tumor with gross cortical bone/marrow, skull base invasion and/or skull base foramen invasion. –T4a Tumor with gross cortical bone/marrow invasion. –T4b Tumor with skull base invasion and/or skull base foramen involvement. N Category N Criteria ENE = extranodal extension. a Reprinted with permission from AJCC: Cutaneous carcinoma of the head and neck. In: Amin MB, Edge SB, Greene FL, et al., eds.:AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 171–81. b A designation of "U" or "L" may be used for any N category to indicate metastasis above the lower border of the cricoid (U) or below the lower border of the cricoid (L). Similarly, clinical and pathological ENE should be recorded as ENE negative or ENE positive. NX Regional lymph nodes cannot be assessed. N0 No regional lymph node metastasis. N1 Metastasis in a single ipsilateral lymph node, ≤3 cm in greatest dimension and ENE negative. N2 Metastasis in a single ipsilateral lymph node, ≤3 cm in greatest dimension and ENE positive;or>3 cm but ≤6 cm in greatest dimension and ENE negative;or metastases in multiple ipsilateral lymph nodes, none >6 cm in greatest dimension and ENE negative;or in bilateral or contralateral lymph node(s), none >6 cm in greatest dimension, ENE negative. –N2a Metastasis in single ipsilateral node ≤3 cm in greatest dimension and ENE positive;or a single ipsilateral node >3 cm but ≤6 cm in greatest dimension and ENE negative. –N2b Metastasis in multiple ipsilateral nodes, none >6 cm in greatest dimension and ENE negative. –N2c Metastasis in bilateral or contralateral lymph node(s), none >6 cm in greatest dimension and ENE negative. N3 Metastasis in a lymph node >6 cm in greatest dimension and ENE negative;or in a single ipsilateral node >3 cm in greatest dimension and ENE positive;or multiple ipsilateral, contralateral, or bilateral nodes, any with ENE-positive status;or a single contralateral node of any size and ENE positive. –N3a Metastasis in a lymph node >6 cm in greatest dimension and ENE negative. –N3b Metastasis in a single ipsilateral node >3 cm in greatest dimension and ENE positive;or multiple ipsilateral, contralateral, or bilateral nodes, any with ENE-positive status;or a single contralateral node of any size and ENE positive. N Category N Criteria ENE = extranodal extension. a Reprinted with permission from AJCC: Cutaneous carcinoma of the head and neck. In: Amin MB, Edge SB, Greene FL, et al., eds.:AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 171–81. b A designation of "U" or "L" may be used for any N category to indicate metastasis above the lower border of the cricoid (U) or below the lower border of the cricoid (L). Similarly, clinical and pathological ENE should be recorded as ENE negative or ENE positive. NX Regional lymph nodes cannot be assessed. N0 No regional lymph node metastasis. N1 Metastasis in a single ipsilateral lymph node, ≤3 cm in greatest dimension and ENE negative. N2 Metastasis in a single ipsilateral node >3 cm but ≤6 cm in greatest dimension and ENE negative;or metastases in multiple ipsilateral lymph nodes, none >6 cm in greatest dimension and ENE negative;or in bilateral and contralateral lymph nodes, none >6 cm in greatest dimension and ENE negative. –N2a Metastasis in a single ipsilateral node >3 cm but ≤6 cm in greatest dimension and ENE negative. –N2b Metastasis in multiple ipsilateral lymph nodes, none >6 cm in greatest dimension and ENE negative. –N2c Metastasis in bilateral or contralateral lymph nodes, none >6 cm in greatest dimension and ENE negative. N3 Metastasis in a lymph node >6 cm in greatest dimension and ENE negative;or metastasis in any node(s) and clinically overt ENE (ENE positive). –N3a Metastasis in a lymph node >6 cm in greatest dimension and ENE negative. –N3b Metastasis in any node(s) and ENE positive. M Category M Criteria a Reprinted with permission from AJCC: Cutaneous carcinoma of the head and neck. In: Amin MB, Edge SB, Greene FL, et al., eds.:AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 171–81. M0 No distant metastasis. M1 Distant metastasis. Stage T N M Illustration M = distant metastasis; N = regional lymph nodes; T = primary tumor. a Reprinted with permission from AJCC: Cutaneous carcinoma of the head and neck. In: Amin MB, Edge SB, Greene FL, et al., eds.:AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 171–81. 0 Tis N0 M0
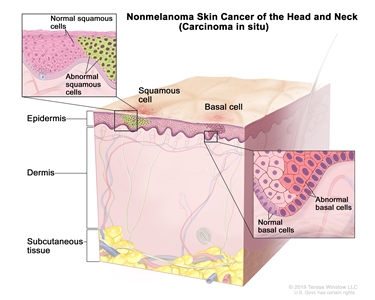
I T1 N0 M0
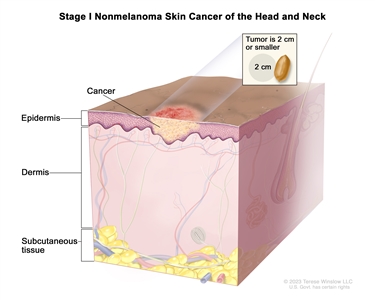
II T2 N0 M0
III T1 N1 M0
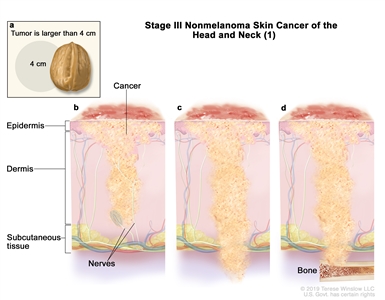
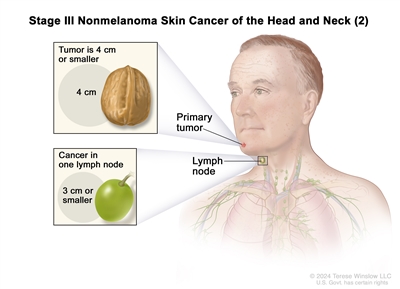
T2 N1 M0 T3 N0 M0 T3 N1 M0 IV T1 N2 M0
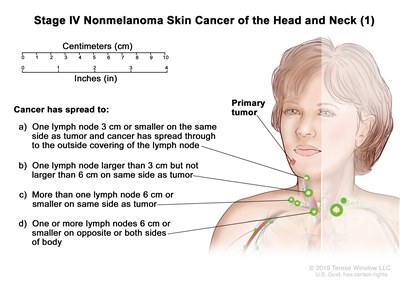
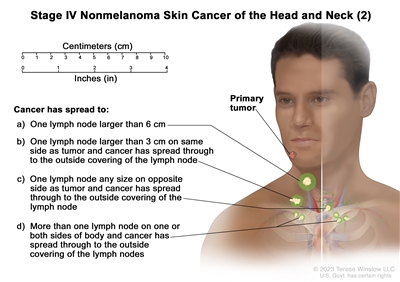
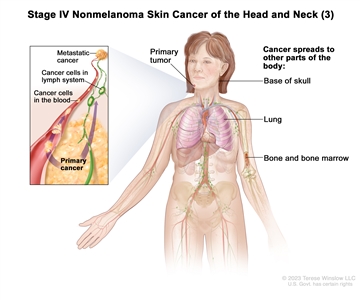
T2 N2 M0 T3 N2 M0 T4 Any N M0 Any T N3 M0 Any T Any N M1
T Category T Criteria a Reprinted with permission from AJCC: Eyelid carcinoma. In: Amin MB, Edge SB, Greene FL, et al., eds.:AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 779–85. TX Primary tumor cannot be assessed. T0 No evidence of primary tumor. Tis Carcinomain situ. T1 Tumor ≤10 mm in greatest dimension. –T1a Tumor does not invade the tarsal plate or eyelid margin. –T1b Tumor invades the tarsal plate or eyelid margin. –T1c Tumor involves full thickness of the eyelid. T2 Tumor >10 mm but ≤20 mm in greatest dimension. –T2a Tumor does not invade the tarsal plate or eyelid margin. –T2b Tumor invades the tarsal plate or eyelid margin. –T2c Tumor involves full thickness of the eyelid. T3 Tumor >20 mm but ≤30 mm in greatest dimension. –T3a Tumor does not invade the tarsal plate or eyelid margin. –T3b Tumor invades the tarsal plate or eyelid margin. –T3c Tumor involves full thickness of the eyelid. T4 Any eyelid tumor that invades adjacent ocular, orbital, or facial structures. –T4a Tumor invades ocular or intraorbital structures. –T4b Tumor invades (or erodes through) the bony walls of the orbit or extends to the paranasal sinuses or invades the lacrimal sac/nasolacrimal duct or brain. N Category N Criteria a Reprinted with permission from AJCC: Eyelid carcinoma. In: Amin MB, Edge SB, Greene FL, et al., eds.:AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 779–85. NX Regional lymph nodes cannot be assessed. N0 No evidence of lymph node involvement. N1 Metastasis in a single ipsilateral regional lymph node, ≤3 cm in greatest dimension. –N1a Metastasis in a single ipsilateral lymph node based on clinical evaluation or imaging findings. –N1b Metastasis in a single ipsilateral lymph node based on lymph node biopsy. N2 Metastasis in a single ipsilateral lymph node, >3 cm in greatest dimension;or in bilateral or contralateral lymph nodes. –N2a Metastasis documented based on clinical evaluation or imaging findings. –N2b Metastasis documented based on microscopic findings on lymph node biopsy. M Category M Criteria a Reprinted with permission from AJCC: Eyelid carcinoma. In: Amin MB, Edge SB, Greene FL, et al., eds.:AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 779–85. M0 No distant metastasis. M1 Distant metastasis. Stage T N M M = distant metastasis; N = regional lymph nodes; T = primary tumor. a Reprinted with permission from AJCC: Eyelid carcinoma. In: Amin MB, Edge SB, Greene FL, et al., eds.:AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 779–85. 0 Tis N0 M0 IA T1 N0 M0 IB T2a N0 M0 IIA T2b–c N0 M0 T3 N0 M0 IIB T4 N0 M0 IIIA Any T N1 M0 IIIB Any T N2 M0 IV Any T Any N M1 Treatment Option Overview
Treatment of Basal Cell Carcinoma of the Skin
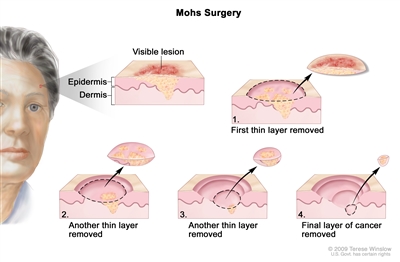
Mohs surgery. A surgical procedure to remove skin cancer in several steps. First, a thin layer of cancerous tissue is removed. Then, a second thin layer of tissue is removed and viewed under a microscope to check for cancer cells. More layers are removed one at a time until the tissue viewed under a microscope shows no remaining cancer. This type of surgery is used to remove as little normal tissue as possible and is often used to remove skin cancer on the face. Treatment of Squamous Cell Carcinoma of the Skin

Mohs surgery. A surgical procedure to remove skin cancer in several steps. First, a thin layer of cancerous tissue is removed. Then, a second thin layer of tissue is removed and viewed under a microscope to check for cancer cells. More layers are removed one at a time until the tissue viewed under a microscope shows no remaining cancer. This type of surgery is used to remove as little normal tissue as possible and is often used to remove skin cancer on the face. Treatment of Actinic Keratosis
Latest Updates to This Summary (03 / 07 / 2025)
About This PDQ Summary
Our Health Library information does not replace the advice of a doctor. Please be advised that this information is made available to assist our patients to learn more about their health. Our providers may not see and/or treat all topics found herein.Skin Cancer Treatment (PDQ®): Treatment - Health Professional Information [NCI]



