Our Health Library information does not replace the advice of a doctor. Please be advised that this information is made available to assist our patients to learn more about their health. Our providers may not see and/or treat all topics found herein. This information is produced and provided by the National Cancer Institute (NCI). The information in this topic may have changed since it was written. For the most current information, contact the National Cancer Institute via the Internet web site at http://cancer.gov or call 1-800-4-CANCER. Endometrial cancer is a disease in which malignant (cancer) cells form in the tissues of the endometrium. The endometrium is the lining of the uterus, a hollow, muscular organ in a woman's pelvis. The uterus is where a fetus grows. In most nonpregnant women, the uterus is about 3 inches long. The lower, narrow end of the uterus is the cervix, which leads to the vagina. Cancer of the endometrium is different from cancer of the muscle of the uterus, which is called sarcoma of the uterus. See the PDQ summary on Uterine Sarcoma Treatment for more information about uterine sarcoma. Obesity and having metabolic syndrome may increase the risk of endometrial cancer. Anything that increases your chance of getting a disease is called a risk factor. Having a risk factor does not mean that you will get cancer; not having risk factors doesn't mean that you will not get cancer. Talk to your doctor if you think you may be at risk for endometrial cancer. Risk factors for endometrial cancer include the following: Older age is the main risk factor for most cancers. The chance of getting cancer increases as you get older. Taking tamoxifen for breast cancer or taking estrogen alone (without progesterone) can increase the risk of endometrial cancer. Endometrial cancer may develop in breast cancer patients who have been treated with tamoxifen. A patient who takes this drug and has abnormal vaginal bleeding should have a follow-up exam and a biopsy of the endometrial lining if needed. Women taking estrogen (a hormone that can affect the growth of some cancers) alone also have an increased risk of endometrial cancer. Taking estrogen combined with progesterone (another hormone) does not increase a woman's risk of endometrial cancer. Signs and symptoms of endometrial cancer include unusual vaginal bleeding or pain in the pelvis. These and other signs and symptoms may be caused by endometrial cancer or by other conditions. Check with your doctor if you have any of the following: Tests that examine the endometrium are used to diagnose endometrial cancer. Because endometrial cancer begins inside the uterus, it does not usually show up in the results of a Pap test. For this reason, a sample of endometrial tissue must be removed and checked under a microscope to look for cancer cells. One of the following procedures may be used: Other tests and procedures used to diagnose endometrial cancer include the following: Certain factors affect prognosis (chance of recovery) and treatment options. The prognosis and treatment options depend on the following: Endometrial cancer can usually be cured because it is usually diagnosed early. After endometrial cancer has been diagnosed, tests are done to find out if cancer cells have spread within the uterus or to other parts of the body. The process used to find out whether the cancer has spread within the uterus or to other parts of the body is called staging. The information gathered from the staging process determines the stage of the disease. It is important to know the stage in order to plan treatment. Certain tests and procedures are used in the staging process. A hysterectomy (an operation in which the uterus is removed) will usually be done to treat endometrial cancer. Tissue samples are taken from the area around the uterus and checked under a microscope for signs of cancer to help find out whether the cancer has spread. The following procedures may be used in the staging process: There are three ways that cancer spreads in the body. Cancer can spread through tissue, the lymph system, and the blood: Cancer may spread from where it began to other parts of the body. When cancer spreads to another part of the body, it is called metastasis. Cancer cells break away from where they began (the primary tumor) and travel through the lymph system or blood. The metastatic tumor is the same type of cancer as the primary tumor. For example, if endometrial cancer spreads to the lung, the cancer cells in the lung are actually endometrial cancer cells. The disease is metastatic endometrial cancer, not lung cancer. The following stages are used for endometrial cancer: Stage I In stage I, cancer is found in the uterus only. Stage I is divided into stages IA and IB, based on how far the cancer has spread. Stage II In stage II, cancer has spread into connective tissue of the cervix, but has not spread outside the uterus. Stage III In stage III, cancer has spread beyond the uterus and cervix, but has not spread beyond the pelvis. Stage III is divided into stages IIIA, IIIB, and IIIC, based on how far the cancer has spread within the pelvis. Stage IV In stage IV, cancer has spread beyond the pelvis. Stage IV is divided into stages IVA and IVB, based on how far the cancer has spread. Endometrial cancer may be grouped for treatment as follows: Low-risk endometrial cancer Grades 1 and 2 tumors are usually considered low-risk. They usually do not spread to other parts of the body. High-risk endometrial cancer Grade 3 tumors are considered high-risk. They often spread to other parts of the body. Uterine papillary serous, clear cell, and carcinosarcoma are three subtypes of endometrial cancer that are considered grade 3. Endometrial cancer can recur (come back) after it has been treated. The cancer may come back in the uterus, the pelvis, in lymph nodes in the abdomen, or in other parts of the body. There are different types of treatment for patients with endometrial cancer. Different types of treatment are available for patients with endometrial cancer. Some treatments are standard (the currently used treatment), and some are being tested in clinical trials. A treatment clinical trial is a research study meant to help improve current treatments or obtain information on new treatments for patients with cancer. When clinical trials show that a new treatment is better than the standard treatment, the new treatment may become the standard treatment. Patients may want to think about taking part in a clinical trial. Some clinical trials are open only to patients who have not started treatment. Five types of standard treatment are used: Surgery Surgery (removing the cancer in an operation) is the most common treatment for endometrial cancer. The following surgical procedures may be used: After the doctor removes all the cancer that can be seen at the time of the surgery, some patients may be given radiation therapy or hormone treatment after surgery to kill any cancer cells that are left. Treatment given after the surgery, to lower the risk that the cancer will come back, is called adjuvant therapy. Radiation therapy Radiation therapy is a cancer treatment that uses high-energy x-rays or other types of radiation to kill cancer cells or keep them from growing. There are two types of radiation therapy: The way the radiation therapy is given depends on the type and stage of the cancer being treated. External and internal radiation therapy are used to treat endometrial cancer, and may also be used as palliative therapy to relieve symptoms and improve quality of life. Chemotherapy Chemotherapy is a cancer treatment that uses drugs to stop the growth of cancer cells, either by killing the cells or by stopping the cells from dividing. When chemotherapy is taken by mouth or injected into a vein or muscle, the drugs enter the bloodstream and can reach cancer cells throughout the body (systemic chemotherapy). When chemotherapy is placed directly into the cerebrospinal fluid, an organ, or a body cavity such as the abdomen, the drugs mainly affect cancer cells in those areas (regional chemotherapy). The way the chemotherapy is given depends on the type and stage of the cancer being treated. Hormone therapy Hormone therapy is a cancer treatment that removes hormones or blocks their action and stops cancer cells from growing. Hormones are substances made by glands in the body and circulated in the bloodstream. Some hormones can cause certain cancers to grow. If tests show that the cancer cells have places where hormones can attach (receptors), drugs, surgery, or radiation therapy is used to reduce the production of hormones or block them from working. Targeted therapy Targeted therapy is a type of treatment that uses drugs or other substances to identify and attack specific cancer cells. Targeted therapies usually cause less harm to normal cells than chemotherapy or radiation therapy do. Monoclonal antibodies, mTOR inhibitors, and signal transduction inhibitors are three types of targeted therapy used to treat endometrial cancer. New types of treatment are being tested in clinical trials. Information about clinical trials is available from the NCI website. Treatment for endometrial cancer may cause side effects. For information about side effects caused by treatment for cancer, visit our Side Effects page. Patients may want to think about taking part in a clinical trial. For some patients, taking part in a clinical trial may be the best treatment choice. Clinical trials are part of the cancer research process. Clinical trials are done to find out if new cancer treatments are safe and effective or better than the standard treatment. Many of today's standard treatments for cancer are based on earlier clinical trials. Patients who take part in a clinical trial may receive the standard treatment or be among the first to receive a new treatment. Patients who take part in clinical trials also help improve the way cancer will be treated in the future. Even when clinical trials do not lead to effective new treatments, they often answer important questions and help move research forward. Patients can enter clinical trials before, during, or after starting their cancer treatment. Some clinical trials only include patients who have not yet received treatment. Other trials test treatments for patients whose cancer has not gotten better. There are also clinical trials that test new ways to stop cancer from recurring (coming back) or reduce the side effects of cancer treatment. Clinical trials are taking place in many parts of the country. Information about clinical trials supported by NCI can be found on NCI's clinical trials search webpage. Clinical trials supported by other organizations can be found on the ClinicalTrials.gov website. Follow-up tests may be needed. As you go through treatment, you will have follow-up tests or check-ups. Some tests that were done to diagnose or stage the cancer may be repeated to see how well the treatment is working. Decisions about whether to continue, change, or stop treatment may be based on the results of these tests. Some of the tests will continue to be done from time to time after treatment has ended. The results of these tests can show if your condition has changed or if the cancer has recurred (come back). For information about the treatments listed below, see the Treatment Option Overview section. Low-risk endometrial cancer (grade 1 or grade 2) Treatment of low-risk stage I endometrial cancer and stage II endometrial cancer may include the following: If cancer has spread to the cervix, a radical hysterectomy with bilateral salpingo-oophorectomy may be done. High-risk endometrial cancer (grade 3) Treatment of high-risk stage I endometrial cancer and stage II endometrial cancer may include the following: Use our clinical trial search to find NCI-supported cancer clinical trials that are accepting patients. You can search for trials based on the type of cancer, the age of the patient, and where the trials are being done. General information about clinical trials is also available. For information about the treatments listed below, see the Treatment Option Overview section. Treatment of stage III endometrial cancer, stage IV endometrial cancer, and recurrent endometrial cancer may include the following: Use our clinical trial search to find NCI-supported cancer clinical trials that are accepting patients. You can search for trials based on the type of cancer, the age of the patient, and where the trials are being done. General information about clinical trials is also available. For more information from the National Cancer Institute about endometrial cancer, see the following: For general cancer information and other resources from the National Cancer Institute, visit: About PDQ Physician Data Query (PDQ) is the National Cancer Institute's (NCI's) comprehensive cancer information database. The PDQ database contains summaries of the latest published information on cancer prevention, detection, genetics, treatment, supportive care, and complementary and alternative medicine. Most summaries come in two versions. The health professional versions have detailed information written in technical language. The patient versions are written in easy-to-understand, nontechnical language. Both versions have cancer information that is accurate and up to date and most versions are also available in Spanish. PDQ is a service of the NCI. The NCI is part of the National Institutes of Health (NIH). NIH is the federal government's center of biomedical research. The PDQ summaries are based on an independent review of the medical literature. They are not policy statements of the NCI or the NIH. Purpose of This Summary This PDQ cancer information summary has current information about the treatment of endometrial cancer. It is meant to inform and help patients, families, and caregivers. It does not give formal guidelines or recommendations for making decisions about health care. Reviewers and Updates Editorial Boards write the PDQ cancer information summaries and keep them up to date. These Boards are made up of experts in cancer treatment and other specialties related to cancer. The summaries are reviewed regularly and changes are made when there is new information. The date on each summary ("Updated") is the date of the most recent change. The information in this patient summary was taken from the health professional version, which is reviewed regularly and updated as needed, by the PDQ Adult Treatment Editorial Board. Clinical Trial Information A clinical trial is a study to answer a scientific question, such as whether one treatment is better than another. Trials are based on past studies and what has been learned in the laboratory. Each trial answers certain scientific questions in order to find new and better ways to help cancer patients. During treatment clinical trials, information is collected about the effects of a new treatment and how well it works. If a clinical trial shows that a new treatment is better than one currently being used, the new treatment may become "standard." Patients may want to think about taking part in a clinical trial. Some clinical trials are open only to patients who have not started treatment. Clinical trials can be found online at NCI's website. For more information, call the Cancer Information Service (CIS), NCI's contact center, at 1-800-4-CANCER (1-800-422-6237). Permission to Use This Summary PDQ is a registered trademark. The content of PDQ documents can be used freely as text. It cannot be identified as an NCI PDQ cancer information summary unless the whole summary is shown and it is updated regularly. However, a user would be allowed to write a sentence such as "NCI's PDQ cancer information summary about breast cancer prevention states the risks in the following way: [include excerpt from the summary]." The best way to cite this PDQ summary is: PDQ® Adult Treatment Editorial Board. PDQ Endometrial Cancer Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/uterine/patient/endometrial-treatment-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389334] Images in this summary are used with permission of the author(s), artist, and/or publisher for use in the PDQ summaries only. If you want to use an image from a PDQ summary and you are not using the whole summary, you must get permission from the owner. It cannot be given by the National Cancer Institute. Information about using the images in this summary, along with many other images related to cancer can be found in Visuals Online. Visuals Online is a collection of more than 3,000 scientific images. Disclaimer The information in these summaries should not be used to make decisions about insurance reimbursement. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page. Contact Us More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website's E-mail Us. Last Revised: 2020-11-13 If you want to know more about cancer and how it is treated, or if you wish to know about clinical trials for your type of cancer, you can call the NCI's Cancer Information Service at 1-800-422-6237, toll free. A trained information specialist can talk with you and answer your questions. This information does not replace the advice of a doctor. Ignite Healthwise, LLC disclaims any warranty or liability for your use of this information. Your use of this information means that you agree to the Terms of Use and Privacy Policy. Learn how we develop our content. Healthwise, Healthwise for every health decision, and the Healthwise logo are trademarks of Ignite Healthwise, LLC.Endometrial Cancer Treatment (PDQ®): Treatment - Patient Information [NCI]
General Information About Endometrial Cancer
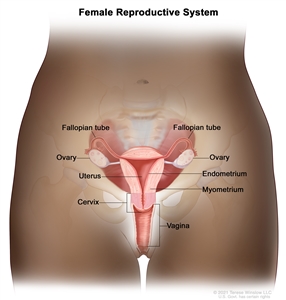
Anatomy of the female reproductive system. The organs in the female reproductive system include the uterus, ovaries, fallopian tubes, cervix, and vagina. The uterus has a muscular outer layer called the myometrium and an inner lining called the endometrium. 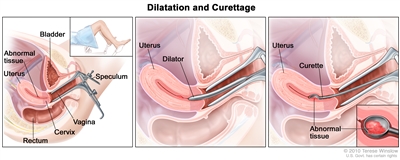
Dilatation and curettage (D and C). A speculum is inserted into the vagina to widen it in order to look at the cervix (first panel). A dilator is used to widen the cervix (middle panel). A curette is put through the cervix into the uterus to scrape out abnormal tissue (last panel).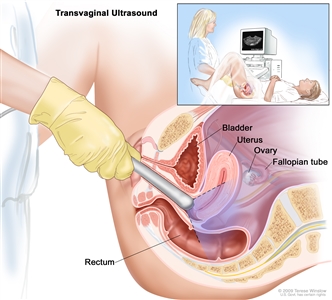
Transvaginal ultrasound. An ultrasound probe connected to a computer is inserted into the vagina and is gently moved to show different organs. The probe bounces sound waves off internal organs and tissues to make echoes that form a sonogram (computer picture).Stages of Endometrial Cancer
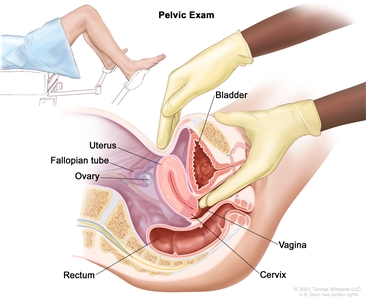
Pelvic exam. A doctor or nurse inserts one or two lubricated, gloved fingers of one hand into the vagina and presses on the lower abdomen with the other hand. This is done to feel the size, shape, and position of the uterus and ovaries. The vagina, cervix, fallopian tubes, and rectum are also checked.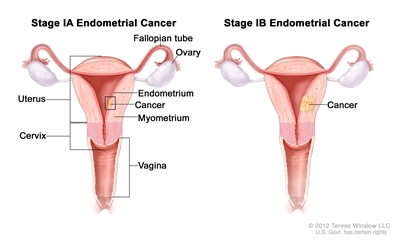
Stage IA and stage IB endometrial cancer. In stage IA, cancer is in the endometrium only or less than halfway through the myometrium (the muscle layer of the uterus). In stage IB, cancer has spread halfway or more into the myometrium.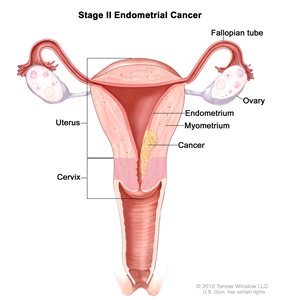
Stage II endometrial cancer. Cancer has spread into connective tissue of the cervix, but has not spread outside the uterus.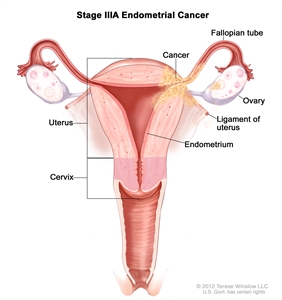
Stage IIIA endometrial cancer. Cancer has spread to the outer layer of the uterus and/or to the fallopian tubes, ovaries, or ligaments of the uterus.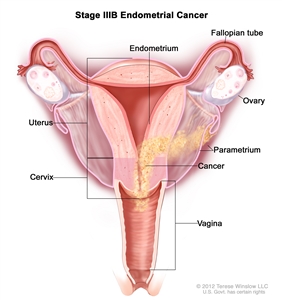
Stage IIIB endometrial cancer. Cancer has spread to the vagina and/or to the parametrium (connective tissue and fat around the uterus and cervix).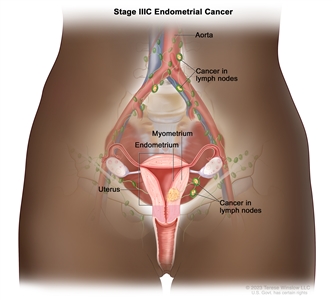
Stage IIIC endometrial cancer. Cancer has spread to lymph nodes in the pelvis and/or around the aorta (the largest artery in the body that carries blood away from the heart).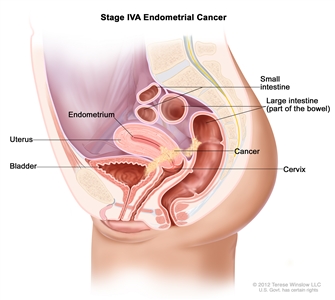
Stage IVA endometrial cancer. Cancer has spread into the bladder and/or bowel.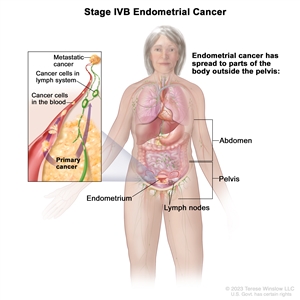
Stage IVB endometrial cancer. The cancer has spread to parts of the body outside the pelvis, including the abdomen and/or lymph nodes in the groin.Treatment Option Overview
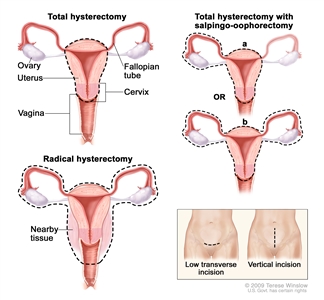
Hysterectomy. The uterus is surgically removed with or without other organs or tissues. In a total hysterectomy, the uterus and cervix are removed. In a total hysterectomy with salpingo-oophorectomy, (a) the uterus plus one (unilateral) ovary and fallopian tube are removed; or (b) the uterus plus both (bilateral) ovaries and fallopian tubes are removed. In a radical hysterectomy, the uterus, cervix, both ovaries, both fallopian tubes, and nearby tissue are removed. These procedures are done using a low transverse incision or a vertical incision.Treatment of Stage I and Stage II Endometrial Cancer
Treatment of Stage III, Stage IV, and Recurrent Endometrial Cancer
To Learn More About Endometrial Cancer
About This PDQ Summary
Our Health Library information does not replace the advice of a doctor. Please be advised that this information is made available to assist our patients to learn more about their health. Our providers may not see and/or treat all topics found herein.Endometrial Cancer Treatment (PDQ®): Treatment - Patient Information [NCI]



