Our Health Library information does not replace the advice of a doctor. Please be advised that this information is made available to assist our patients to learn more about their health. Our providers may not see and/or treat all topics found herein. This information is produced and provided by the National Cancer Institute (NCI). The information in this topic may have changed since it was written. For the most current information, contact the National Cancer Institute via the Internet web site at http://cancer.gov or call 1-800-4-CANCER. The American Cancer Society estimates that 618,120 Americans will die of cancer in 2025.[1] Anticipating the end of life (EOL) and making health care decisions about appropriate or preferred treatment or care near the EOL is intellectually challenging and emotionally distressing for patients with advanced cancer, their families and friends, oncology clinicians, and other professional caregivers. However, the adverse consequences of failing to plan for the transition to EOL care include the following: Oncologists and patients often avoid or delay planning for the EOL until the final weeks or days of life because of many potential factors. These factors can be at the individual, family, or societal levels. Evidence suggests, however, that many of these factors are not truly barriers and can be overcome. The purpose of this summary is to review the evidence surrounding conversations about EOL care in advanced cancer to inform providers, patients, and families about the transition to compassionate and effective EOL care. References: Patients with advanced cancer, their family and friends, and oncology clinicians often are faced with treatment decisions that profoundly affect the patient's quality of life (QOL). Oncology clinicians are obligated to explore, with the patient and family, the potential impact of continued disease-directed treatments or care directed at the patient's symptoms and QOL. This section summarizes information that will allow oncology clinicians and patients with advanced cancer to create a plan of care to improve QOL at the end of life (EOL) by making informed choices about the potential harms of continued aggressive treatment and the potential benefits of palliative or hospice care. This section: In addition, information about outcomes associated with cardiopulmonary resuscitation (CPR) and admission to the intensive care unit (ICU) at the EOL will allow the oncology clinician to better present options to patients with advanced cancer who are near the EOL. Quality of EOL Care Questions relevant to measuring the quality of EOL care in patients with advanced cancer include the following:[1] The patient perspective Surveys and interviews of patients with life-threatening illnesses, not restricted to cancer, can contribute to the understanding of what constitutes high-quality EOL care. One group of researchers proposed that patients value five main domains of care near the EOL:[2] A 2011 prospective study of QOL in a cohort of patients with advanced cancer seen in outpatient medical oncology clinics provides additional insight into the patient's perspective of what contributes to a good QOL.[3] The median survival of the cohort was 10 months, so the results may not reflect the situation for patients closer to death. Nonetheless, the strongest predictors of QOL were the following: Patients who were waiting for a new treatment had worse emotional well-being. This experience suggests that QOL is related to factors such as disease progression and its complications, and to patients' goals relative to any treatment they are receiving.[3] In addition, other researchers have reported that family caregivers often report worse scores than patients with advanced cancer in self-assessments of the patients' QOL.[4] Indicators of EOL care quality A variety of indicators have been proposed to measure the quality of EOL care in patients with advanced cancer. Several salient criticisms of the proposed indicators include the following: Nonetheless, studying indicators over time or between different geographic regions, health systems, or subspecialties can provide important insights into quality EOL care. Trends over time in indicators of EOL care quality Multiple reports are relevant to understanding trends in EOL care quality indicators over time for a variety of cancers. The following observations are supported by a 2004 analysis,[6] with additional supporting citations indicated when relevant: Regional variations in indicators of EOL care quality Regional variations in rates of utilization of health care resources near the EOL are of interest because the differences are rarely associated with improved outcomes. While initial findings focused on differences between geographic regions of the United States, subsequent studies have demonstrated potentially meaningful differences between or within health systems.[10] A brief summary of some notable variations follows. In the opinion of the authors, the observed regional variations were too large to be accounted for by racial or ethnic preferences or illness levels. Factors correlated with the aggressiveness of EOL care include the availability of resources such as the supply of ICU beds or imaging equipment, the number of doctors involved in each patient's care, and the treatment setting itself.[12] Factors associated with variations in EOL care Availability of medical specialists, number of hospital beds, physician, and health system characteristics are well-established factors associated with increased expenditures in the final 6 months of life. A 2011 study [13] used data from decedents enrolled in the Health and Retirement Study, Medicare, and the Dartmouth Atlas to identify patient-level factors that may contribute to regional variations. The following factors were associated with higher expenditures, even after controlling for regional characteristics: Patient-level factors accounted for only 10% of variations. EOL practice patterns and number of hospital beds per capita were also associated with higher expenditures. Advance care planning did not influence expenditures.[13] A potential explanation for regional variations in EOL care is regional variations in patient preferences. The evidence, however, is conflicting. The Good Death The health care provider perspective The concept of a good death is a controversial but potentially useful construct for the oncology clinician to more clearly formulate the goals of timely, compassionate, and effective EOL care. In a 2003 BMJ article about caring for the dying patient, the authors proposed that there were sufficient evidence-based guidelines to facilitate a good death. A commentary that accompanied the article stated, "Nor can professional education convey adequately just how important it is for individuals, both at the time and afterwards, to go through the death of someone they love feeling that they are experiencing a ‘good death.'"[16] Several salient points were made in subsequent letters to the editor: The patient perspective A landmark study of patients, families, and health care providers [17] surveyed seriously ill patients, bereaved caregivers, physicians, and other health care providers about what matters at the EOL. Respondents scored the importance of 44 attributes identified in previous interviews and focus groups in which participants were asked to define a good death. There was broad agreement about control of pain and other symptoms, communication, and preparation for death. However, certain attributes, such as not being a burden to one's family or society or being at peace with God, were ranked as important by patients but less so by physicians. A population-based study in the Netherlands demonstrated a correlation between the respondents' positive attitudes toward the concept of a good death and preferences that could potentially influence EOL decision making.[18] Patients with advanced cancer may desire opportunities to prepare for the EOL. A survey of 469 patients who participated in a cluster-randomized trial of early palliative care [19,20] reported that better preparation was associated with higher scores related to the quality of communication with clinicians, older age, living alone, fewer symptoms, and spiritual well-being. Interestingly, 31% of respondents were worried about their family's future coping. The results highlight the importance of offering patients an opportunity to discuss preparation for the EOL early and the fact that patients who favorably rate the quality of communication with their physicians feel more prepared. The caregiver perspective In one study,[21] formal and informal caregivers of 396 patients who died from advanced cancer were interviewed about the decedent's QOL in the last week of life. The QOL was correlated with a variety of factors obtained via baseline interviews of decedents at the time of enrollment into the study and survey measures of coping styles, religious coping, religiousness/spirituality, and EOL preferences. Most variances in the QOL at the EOL were unexplained. However, factors negatively correlated with QOL included the following: Conversely, factors correlated with a higher QOL included the following: Outcomes After Potentially Life-Prolonging Interventions CPR CPR was initially developed to restore circulation in patients with predominantly cardiac insults. The outcomes after inpatient CPR in older adults have not improved significantly, in part because of the use of CPR in patients with comorbid life-threatening illnesses. Addressing the limited benefit of CPR during the transition to EOL care is made more difficult because in the United States, patients will undergo CPR unless there is an established DNR order in the medical chart to countermand the procedure. The following evidence demonstrates the limited value of CPR in patients with advanced cancer: Admission to an ICU In addition to CPR, patients may require mechanical ventilation or admission to an ICU. Outcomes are poor for patients with advanced cancer. One study reported that the median survival of 212 patients with advanced cancer (who were referred to a phase I trial) was 3.2 weeks.[26] Patients who required CPR had a median survival of 1 day. The underlying diagnosis, however, may be a critical variable in predicting patient outcome. Patients with hematologic malignancies may do better than patients with solid tumors. For example, one study reported that patients with newly diagnosed hematologic malignancies had a 60.7% chance of surviving to be discharged and a 1-year survival rate of 43.3%.[27] Multivariate analysis demonstrated that remission status and early admission to the ICU were favorable risk factors. References: Overview One interpretation of the evidence, summarized in the Quality of End-of-Life Care in Patients With Advanced Cancer section, is that patients with advanced cancer too often receive burdensome and potentially harmful treatments without much chance of benefit and to the detriment of receiving purposeful end-of-life (EOL) care. Studies of patients with advanced cancer have identified factors that can influence EOL health care decisions and outcomes. This section provides the oncology clinician with insights about potentially influential factors that may lead to more effective interactions with the patient in planning the transition to EOL care. Several notes of caution about the cited studies, however, are highlighted in the following: Cited Studies Three very large, comprehensive studies provide information for characterizing the relationships among markers of quality communication, decision making, health care decisions, and outcomes in patients with advanced cancer. These studies are described here, and results are integrated into subsequent sections. Phase I of SUPPORT confirmed significant shortcomings in patient-physician communication.[2,3] Phase II demonstrated that the nurse-led intervention did not increase discussions about CPR preferences or concordance between patients and physicians about patients' CPR preferences. It also did not decrease the number of days spent in the intensive care unit (ICU), frequency of mechanical ventilation, or level of pain. Patient Demographics The goal of planning the transition to EOL care in a deliberate and thoughtful manner is to increase the likelihood that a person with advanced cancer will receive high-quality EOL care consistent with their informed preferences. A variety of patient characteristics influence the interaction with the oncology clinician and the patient's decisions or outcomes. The following section highlights representative results of studies of patient demographics and other factors. Age Cancer is a disease of older adults.[7] It is important to remember, however, that there are probably differences in different cohorts of older adults. Gender Race Socioeconomic status Patient Understanding of Prognosis Multiple studies have demonstrated correlations between patients' understanding of their prognoses and health care decisions, medical outcomes, or psychological adjustment near the EOL. However, differences in study methodology, patient populations, measures of prognostic understanding, and the end point studied as the primary outcome preclude definitive conclusions about the relevance of the correlations. Furthermore, causality can only be inferred, given the cross-sectional nature of most studies. Nonetheless, a summary of the published data organized by the measure of prognostic understanding may provide insight into the decision-making processes of patients with advanced cancer. Patient Preferences Respect for patient preferences is essential to high-quality cancer care and to protecting patient autonomy. Patients with advanced cancer who had an opportunity to discuss their EOL preferences were more likely to receive care consistent with their wishes.[26] As it stands, evidence suggests that oncology clinicians often do not elicit or clarify patient preferences and, ultimately, fail to provide care consistent with their preferences. For example, a 2011 study [28] of 128 patients with newly diagnosed advanced-stage lung cancer found that: The final observation highlights the fundamental question of how oncology clinicians should elicit patient preferences. While direct questioning may seem to be the most straightforward approach, a study of two single-item preference measures demonstrated that the decision-making preferences of patients appear to differ on the basis of what measure was utilized.[29] Although the optimal way to elicit patient preferences is uncertain, a few studies have shed light on this topic. Patients with advanced cancer have several preferences of potential significance to planning the transition to EOL care, including: A discussion about preferences is complicated. Preferences may be narrowly construed or may reflect the fundamental values of an individual. In a study of 337 older patients' attitudes about using advance directives to manage EOL care,[30] 85% of respondents believed it was definitely (55%) or somewhat (30%) necessary to record their wishes in advance directives. However, most (80%) preferred either general value and goal statements or both precise and general directions. There was a strong preference for discussion among surrogate decision makers on behalf of the incapacitated person.[30] In a 2020 survey study at Johns Hopkins Hospital, investigators interviewed 200 surgical and medical oncology inpatients about their preferences for discussions about advance directives, which their survey defined as "a document or paper that tells your care providers about the kind of medical treatment you would want if you were very sick and could not make those decisions for yourself."[31] They found that: Preference for information about prognosis Patients with life-limiting illnesses desire information about prognosis,[32] believe that such information may be provided without compromising hope,[33] and prefer that oncologists inquire about their preferences for such information.[34] Younger patient age, female sex, and a shorter life expectancy as perceived by the patient correlate with increased information needs.[8] Preference for decision-making role A variety of measures assess patients' preferences for a decision-making role. The Control Preference Scale [35] asks patients to select one of five statements that best reflects their approaches; results are coded active ("I prefer to make the final selection"), collaborative ("I prefer that my doctor and I share responsibility"), or passive ("I prefer that my doctor makes the final decision"). The available information suggests that preferences vary widely. It is unclear to what extent this variation in preferences is due to differences in disease, different types of patients, or methodological differences. Preference for palliative chemotherapy Several studies have demonstrated that most patients prefer chemotherapy before consultation with a medical oncologist.[38] For example, a group of Dutch researchers assessed the decision-making process of 140 patients with metastatic cancer for whom palliative chemotherapy or best supportive care were reasonable options.[39] Before the consultation, 68% of patients expressed a preference for chemotherapy. Seventy-eight percent of patients eventually chose chemotherapy. The only patient characteristic associated with preference for treatment was younger age. The strongest predictor of treatment choice was the preconsultation preference. The preference for chemotherapy may relate in part to the observations that patients are not fully informed, or they reject the information or reinterpret it to fit their perspectives. In addition, patients may value survival more and QOL less than oncology clinicians anticipate.[40] Preference for QOL or length of life As discussed in the Patient Preferences section, patient preferences may be narrowly or broadly construed. Preferences that are foundational to more specific preferences might be better considered as patient values.[41] In addition to the preference being measured, the methods of measuring preferences vary significantly. The interested reader may consult a 2012 review [42] for a discussion of the different instruments. Notable observations include the following: Patient Goals of Care Discussions about goals of care with advanced-cancer patients are considered to be a critical component of planning the transition to EOL care. However, the definitions of goals of care vary significantly in the relevant literature. Before discussing observations related to goals of care with patients with advanced cancer (and other life-limiting illnesses), it is important to consider whether to distinguish goals of treatment from goals of care. Another perspective is that there is a continuum of goals and that the purpose of conversations about goals of care is to help patients identify alternatives for achieving their goals. A structured literature review and a qualitative analysis of palliative care consultations about goals of care support this perspective, as summarized below: At present, there are no data on the positive or negative influence of discussions about goals of care on EOL outcomes of patients with advanced cancer. Religious and Spiritual Beliefs and Values of Patients Patient religiosity and the provision of spiritual care consistent with a patient's preference have been correlated with EOL outcomes. A series of reports from CwC [49,50,51] found that approximately half of patients with serious or life-threatening illnesses indicated that religious or spiritual beliefs were important to them, as measured by higher scores on the Positive Religious Coping Scales subscale of the RCOPE, a measure of religious coping.[52] RCOPE scores were significantly higher among African American and Hispanic patients. In analyses adjusted for demographic differences, higher levels of positive religious coping were significantly related to the receipt of mechanical ventilation, compared with low levels of religious coping (11.3% vs. 3.6%) and intensive life-prolonging care during the last week of life (13.6% vs. 4.2%).[49] The degree to which the care facility addressed religious and spiritual concerns may substantially affect this relationship. Patients who reported that their spiritual needs were substantially supported by the medical team (26.3%) were significantly more likely to receive hospice care and less likely to receive aggressive care; they also reported higher QOL. Pastoral care, received by about 46% of patients, did not affect receipt of such services, but did affect QOL just before death.[50] Patient-Oncologist EOL Discussions Recall of EOL discussions Recall of EOL discussions influences the EOL health care decisions and outcomes of patients with advanced cancer. A total of 123 of 332 patients (37%) enrolled in CwC answered affirmatively when asked, "Have you and your doctors discussed any particular wishes you have about the care you would want to receive if you were dying?"[4] Recall of EOL discussions was associated with lower rates of mechanical ventilation, resuscitation, or ICU admission and earlier referral to hospice. A subsequent analysis reported on the treatment preferences of 325 patients who died while enrolled in CwC.[26] Seventy-two percent of patients with advanced cancer preferred treatment focused on comfort, answering affirmatively to the question, "If you could choose, would you prefer a plan of care that focused on relieving pain and discomfort, even if this meant not living as long?"; 28% preferred life-extending treatment. Sixty-eight percent received EOL care consistent with baseline preferences. The likelihood of receiving care consistent with preferences was increased if the patient reported an EOL discussion with a physician (OR, 2.26; P < .0001) or was aware of being terminally ill (OR, 3.94; P = .0005). The Nature of the Decision Decision to receive cancer-directed therapy Patients with advanced cancer frequently receive multiple regimens of chemotherapy over the course of their treatment. Whether the decision involves first-line or second-line treatment for advanced disease may influence the decision-making process. One group of investigators conducted 117 semistructured interviews of 102 women with advanced breast cancer who were receiving first-line (n = 70) or second-line (n = 47) palliative chemotherapy.[36] Women in the second-line cohort were more likely than women in the first-line cohort to explain their decision to undergo chemotherapy because of the hope that it offered (43% vs. 19%; P = .006) and to report taking an active role in the decision process. Another research group demonstrated that physicians exerted greater control over decisions when evidence for or against a treatment was less certain or if the cancer was advanced.[53] Thus, the patient's rationale for treatment and the relevance of the oncologist's perspective change with the nature of the decision. Decision to limit treatment Most deaths resulting from advanced cancer are preceded by decisions to limit treatment. Given the prevalence, importance, and challenges of these decisions, however, there is relatively little information about how patient preferences are considered during decision making. Using researchers embedded in health care teams, one group of investigators characterized the deliberations about limiting potentially life-prolonging treatments for 76 hospitalized patients with incurable cancer.[54] Two-thirds of the patients preferred comfort care, and one-third desired life-prolonging therapy. Patient preferences for comfort were more often in line with oncologists' preferences than were patient preferences for life-prolongation (91.4% vs. 46.7%; P = .001). Patients were involved in decisions to limit treatment only half of the time. Age, performance status, or decisional capacity did not influence the rate of patient involvement; agreement with oncologist preferences was the main predictor. Thus, decisions to limit treatment may reflect a shared perception rather than a shared decision-making process. References: The preferences of patients with advanced cancer should, in large part, determine the care they receive. However, evidence suggests that patients lack sufficient opportunity to develop informed preferences and, as a result, may seek care that is potentially inconsistent with their personal values and goals. For more information, see the Factors That Influence End-of-Life Care Decisions and Outcomes section. This section identifies potential barriers that may prevent a patient with advanced cancer and his or her oncologist from discussing prognosis, goals, options, and preferences.[1] The information will allow the oncology clinician to develop the strategies needed to approach these challenging discussions more deliberately. Potential barriers include the following: Patients' Interpretations of Prognostic Information A consistent finding over the last two decades is that patients with advanced cancer are typically overly optimistic about their life expectancies or the potential for cure with cancer-directed therapies. There are many potential barriers to a more accurate understanding of prognosis, including poor communication by oncology clinicians. However, patients also interpret information for reasons unrelated to the quality of communication. The perspectives of patients with advanced cancer who enroll in phase I clinical trials or surrogate decision makers for patients in intensive care units (ICUs) provide some insights into why advanced cancer patients might misinterpret prognostic information. Lack of Agreement Between Patients and Oncologists Multiple conversations between patients with advanced cancer and their oncologists should lead to an understanding about prognoses, goals, preferences, options, and the decision-making process. However, evidence suggests that patients and oncologists frequently do not reach the same conclusions about these issues. How this lack of agreement affects the transition to EOL care is not certain. However, disagreement with or misperceptions about goals of treatment, for example, are unlikely to contribute positively to the timely planning for the transition to EOL care. Understanding of prognosis One group of investigators analyzed the prognostic estimates of 917 adults with metastatic colorectal or lung cancer who were enrolled in the Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments (SUPPORT) and their physicians.[7] There were three notable findings: The poor concordance between patients and oncology clinicians has been observed in a diverse range of patients, including patients with acute myeloid leukemia [8] or those considering allogeneic stem cell transplantation.[9] For more information, see the Patients' Interpretations of Prognostic Information section. Discordance about life expectancy between patients with advanced cancer and their oncologists may be explained by poor communication or factors independent of oncologists' prognostic estimates. One group of researchers attempted to clarify the source of prognostic discordance by surveying 236 patients with advanced cancer who participated in a randomized clinical trial of a communication intervention.[10] The patients and their 38 oncologists were asked to provide an estimate of the chance that the patients would be alive in 2 years. The responses were fixed choices of 100%, about 90%, about 75%, about 50%, about 25%, about 10%, and 0%. Discordance was defined as being more than one response category divergent. As expected, most patient-oncologist dyads were discordant (161 of 236 ratings [68%]; 95% confidence interval [CI], 62%–75%). Almost all discordant patients expressed a more-optimistic prognosis than their oncologists (155 of 161 patients [96%]).[10] The investigators also asked patients to provide the estimate they believed their oncologists would provide. The response categories and definition of discordance were identical. Of the 161 patients who were discordant with their oncologists, 144 (89%) were unaware of the discrepancy. Non-White patients were more likely to provide discordant prognostic expectations. Patient income, education level, sex, or level of recalled prognostic communication did not correlate with discordance. These results support the belief that prognostic discordance reflects inadequate communication of prognostic estimates. However, patients' expressions of life expectancy may also reflect the need for optimism or the belief in unique personal characteristics.[6] Goals of treatment Patients and oncologists frequently do not share an understanding of the goals of cancer treatment. One group of researchers reported that in 25% of the advanced-cancer patient–physician dyads surveyed, the patient thought the goal of care was to cure disease when it was not.[11] Younger patients, patients who were native English speakers, and patients who received written information were more likely to agree with their oncologists about the intent of chemotherapy. Another group of researchers reported that 64% of patients with advanced-stage lung cancer did not understand that the radiation therapy prescribed was not curative.[3] A study of 206 cancer patients and their 11 oncologists sought to identify how well the oncologists understood their patients' goals of care, defined as preference for quality of life versus preference for length of life.[12] The investigators interviewed the oncologists and patients at baseline and every 3 months for 15 months (or until the patient died or enrolled in hospice). The participants were shown a 100-point visual analogue scale with "quality of life is all that matters" at one end of the scale (scored as 0) and "length of life is all that matters" at the other end of the scale (scored as 100). Patients and their oncologists were asked to rate their answers on the scale in response to one of the two questions, "Regarding your care, what is most important to you right now?" (patients) and "Regarding the care of this patient, what do you think is most important to the patient right now?" (oncologists). Participants who scored between 0 and 30 were classified as having a high goal for comfort, while those who scored between 70 and 100 were deemed to have a high goal for survival. The patient-oncologist dyad was considered to be closely aligned if they both scored within the same 30-point windows for a high goal of either comfort (scored 0–30) or survival (scored 70–100). However, at the last interview prior to the patient's death, 76.7% of the patient-oncologist dyads were not in close alignment.[12] Topics recalled from consultation Researchers reported that patients and oncologists frequently recalled different components of communication and decision making from discussions of phase I trials.[13] For example, 13 of 17 participating oncologists mentioned prognosis in fewer than 50% of consultations. Although oncologists frequently reported discussing prognosis, only 12% of patients and 20% of independent researchers who coded the recorded consultations agreed. There was better agreement about unknown adverse events and the voluntary nature of trial enrollment. Patient preference for decision-making role Oncology clinicians frequently do not correctly identify patient preferences for decision-making roles. Results from a few Illustrative studies are summarized below. Assessment of patient performance status Physicians evaluate patient performance status (PS) in determining prognosis and in making treatment decisions. Investigators compared the Eastern Cooperative Oncology Group PS reported by physicians and 1,636 patients who had advanced lung or colorectal cancers.[16] They demonstrated 56.6% disagreement between patients and physicians about PS; more optimism by physicians about PS (mean, 0.91 vs. 1.30); and association of disagreement with an increased risk of death. Patient preference for cardiopulmonary resuscitation The initial phase of SUPPORT demonstrated that only 47% of physicians knew when their patients wanted to avoid cardiopulmonary resuscitation (CPR).[17] In a subsequent analysis, researchers studied 520 patients with metastatic colorectal cancer.[18] Information about CPR preferences for 339 of the patients was available: 223 (63%) wanted CPR; however, physicians incorrectly identified patient preference in 30% of cases. The preference for CPR was stronger among patients who had a more optimistic sense of the likelihood of survival at 2 months. Other investigators found similar results in a group of patients with lung disease.[19] Physicians frequently misunderstood patients' preferences (25%–40% of dyads). The lack of agreement was greater if patients wished to avoid resuscitation. Neither study reported the effect of the disagreement on patient outcomes. Oncologist Communication Behaviors This section summarizes the evidence that identifies deficits in the communication behaviors of oncologists treating patients who have advanced cancer. This evidence strongly suggests that doctor-patient communication frequently does not fully support informed or shared decision making. This information will allow oncology clinicians to reflect on their communication habits and consider modifying impediments to the timely planning for the transition to EOL care. Observational studies of patient-oncologist communication Investigators reported a study of audiotaped consultations between one of nine oncologists and each of 118 patients with advanced cancer.[20] They devised a coding system to assess how frequently essential information (e.g., aim of treatment, incurability of cancer, risks of treatment, information about life expectancy, and alternative treatment options) was disclosed and how much the doctor facilitated the patient's participation in making decisions. A summary of results follows, with independent observations cited as appropriate: Additional deficits in oncologist communication behaviors include the following: Oncologist self-reported practices in prognostic communication There is evidence that physicians' attitudes toward prognostic communication influence patients' prognostic awareness. In an analysis of physician surveys from the Cancer Care Outcomes Research and Surveillance (CanCORS) Consortium,[23] investigators reported that patients with metastatic lung or colorectal cancer were more likely to have an accurate prognostic awareness if their most important doctor reported discussing prognosis earlier rather than waiting for deterioration (18.5% vs. 7.6%; odds ratio, 3.23; 95% confidence interval, 1.39–7.52; P = .006). Thus, understanding the factors that influence oncologists' attitudes is relevant to improving prognostic communication. Two additional surveys of American oncologists and communication about prognosis have been published. One study analyzed the survey responses of 729 oncologists (64% response rate).[24] Almost all (98%) indicated they would disclose a terminal prognosis, but 48% indicated they would do so only when the patient's preference for disclosure of prognosis was known. Fewer than half (43%) of the oncologists always provided an estimate of time until death. Three-quarters of them indicated they had not received formal training in communication of terminal prognoses; 96% thought training should be mandatory. Another study reported that 65% of physicians surveyed discussed prognosis immediately with asymptomatic patients who had advanced cancer and anticipated life expectancies of 4 to 6 months.[25] However, fewer physicians would immediately discuss resuscitation preference (44%), hospice (26%), or preferred site of death (21%), with most physicians waiting for patient symptoms to appear or until there were no more treatments to offer. Younger physicians, surgeons, and oncologists were more likely than noncancer specialists to discuss prognosis. Oncologists' Misconceptions About the Harm of EOL Discussions Oncologists cite several reasons for their reluctance to engage in EOL discussions. However, several studies have provided evidence that many of the concerns—e.g., causing psychological harm or destroying hope—are not valid.[26][Level of evidence: II] On the contrary, EOL conversations are not psychologically harmful and may improve psychological adjustment, while explicit information about prognosis does not necessarily compromise a patient's sense of hope. In another study, men with advanced cancer who estimated a lower likelihood of survival at 6 months had increased levels of anxiety and depression.[28] However, men who reported having a full discussion about prognosis with their oncologist had less depression and similar levels of anxiety. Thus, discussions about prognosis moderated the relationships with anxiety and depression and may have facilitated long-term psychological adjustment. Oncologist Attitudes and Preferences Studies strongly suggest that oncologists' attitudes and preferences influence their communication and decision-making behaviors in a manner that might change patients' EOL decisions. One study found that hospital staff attributed variations in aggressiveness of care near the EOL to physician characteristics, including physician beliefs, attitudes, and socialization within the practice of medicine.[30] As a result, communication about advance directives, prognosis, limiting treatment near the EOL, and acknowledgment that the patient was dying varied significantly among physicians. A 2011 study demonstrated that physicians may recommend different treatments for patients than they would choose for themselves.[31] This section encourages oncology clinicians to reflect on these biases and how to minimize their effects on communication behaviors. Oncologist preferences for noncurative treatments Oncology clinicians influence patients' understanding of treatment preferences. Dutch researchers surveyed medical oncologists about their preferences for palliative (noncurative) chemotherapy or observation using case vignettes.[32] The gender, age, and employment status of the oncologist and type of hospital did not influence preferences for chemotherapy. However, older patients or patients without a stated preference were less likely to receive chemotherapy. There was a greater preference for chemotherapy if the anticipated survival gain was at least 3 months, if the treatment was mildly toxic, or if symptoms of disease progression were identified. These findings are consistent with a study of U.S. oncologists that demonstrated a preference for life-sustaining treatment rather than comfort care.[33] Oncologists and shared decision making In surveys, oncologists are broadly supportive of the concept of shared decision making. However, empirical research demonstrates that oncologists' communication behaviors frequently do not support shared decision making. One group of investigators interviewed Australian cancer specialists about their inclusion of patients in decision making and identified several factors that influence patient involvement.[34] Disease-related factors included: In addition, public perception of the disease and whether there was a clear best treatment option were relevant. Cancer specialists were more likely to include patients in decision making when the disease stage was advanced and treatment options were less certain. Patient characteristics that decreased doctors' efforts to involve the patient included: The doctors were aware of patient preferences for involvement but felt most patients deferred to their expertise. Furthermore, few physicians had a validated approach to determine patient preferences. Oncologist attitudes toward EOL care Attitudes toward EOL care may also influence the communication and decision-making behaviors of oncologists. In a qualitative study of 18 academic oncologists who were asked to reflect on recent patient deaths, one group of researchers reported that oncologists who viewed EOL care as an important part of their job reported increased job satisfaction.[35] Other investigators reported on a survey of Japanese oncologists to assess the level of burden experienced by oncologists when recommending discontinuation of anticancer treatment.[36] Forty-seven percent reported high levels of burden. Multivariate analysis of determinants of the sense of burden identified the following covariates: Reimbursement for Chemotherapy and Practice Economics Before 2003, reimbursement for chemotherapy greatly exceeded acquisition costs for medical oncologists. Although the profit margin for chemotherapy has decreased, the treatment remains a significant source of oncologists' income. Researchers demonstrated that physicians' decisions to prescribe chemotherapy for patients with advanced cancer was not affected by reimbursement rates, but more costly regimens were more likely with higher rates of reimbursement.[37] Additional results are summarized below: Uncertainty About Options Other Than Disease-Directed Treatments A final barrier to planning the transition to EOL care may be confusing language when patients begin to ponder forgoing resuscitation and other life-prolonging interventions. On the basis of their experiences or understanding, oncology clinicians, patients, and families assign different—and often valid—meanings to terms such as supportive or palliative. A 2013 systematic review of the literature found widespread inconsistencies in the definitions of supportive care, palliative care, and hospice.[40] For term definitions and a discussion of clearly communicating the purpose of each level of care for patients with advanced cancer, see the Factors That Influence End-of-Life Care Decisions and Outcomes section. References: Importance of a Name Surveys have identified the term palliative care as a potential impediment to referral to a palliative care clinic.[1] A 2013 report from a single institution that changed the name of its palliative care service to supportive care demonstrated that outpatients were referred sooner after first hospital registration (median, 9.2 months vs. 13.2 months; P < .001) and sooner after the first diagnosis of advanced cancer (median, 5.2 months vs. 6.9 months; P < .001).[2] Although it is unclear how patients interpret these various terms, investigators in a 2018 study who offered breast cancer patients written information about supportive care, palliative care, and/or hospice care found that patients reported more interest in, and were more likely to accept articles about, supportive care versus hospice or palliative care.[3][Level of evidence: II] Oncologists' attitudes toward palliative care The European Society of Medical Oncology surveyed its members about their attitudes toward and involvement in palliative care for patients with advanced cancer.[4] Eighty-eight percent of respondents endorsed the belief that medical oncologists should coordinate end-of-life (EOL) care, but 42% felt they were inadequately trained for the task. Relatively few respondents collaborated with a palliative care specialist (35%) or inpatient hospice services (26%). Public attitudes toward palliative care Public attitudes toward palliative care depend on the description of the service. A 2011 public opinion poll of more than 100 consumers found a lack of knowledge about palliative care among more than 75% of interviewees.[5] After the opinion poll was taken, the definition of palliative care was revised for the public. In the new definition, palliative care is described as "specialized medical care for people with serious illnesses." The focus of care is defined as relief from pain and other symptoms, regardless of diagnosis. Included in the definition are patient and family quality of life (QOL), the interdisciplinary team of specialists, and the statement that palliative care is appropriate at any age, at any stage of illness, and in conjunction with curative treatment.[5] Best Supportive Care The ability to safely administer multiple cycles of chemotherapy depends on a range of interventions to minimize adverse effects. Most oncologists recognize that anticipating, recognizing, and responding to common adverse events with antiemetics, hematopoietic growth factors to reduce the occurrence of red blood cell transfusions or risks of neutropenia-associated infections, transfusions, medications to alleviate anxiety or depression, and analgesics for pain are mandatory skill sets in medical oncology. In this context, supportive care is an adjunct to the goal of maximizing the benefits of disease-directed treatment while minimizing potential decline in a patient's health-related QOL. Three points about the term supportive care are relevant to this section: Palliative Care Palliative care is an interdisciplinary model of care focused on patients with serious or life-threatening illnesses and their families. The goals of palliative care are to: Palliative care is generally provided by physicians, advance practice clinicians, nurses, social workers, and/or chaplains. Palliative care teams typically focus on the following: Palliative care is both a philosophy of care and an organized, highly structured system for delivering care. Palliative care goes beyond the traditional disease model to include goals of care such as the following: Palliative care can be delivered concurrently with life-prolonging care or as the main focus of care. For more information, see the Integrating Palliative Care and Conventional Cancer Care section. The term palliative care has often been used to communicate the noncurative intent of chemotherapy for patients with advanced cancer or to signal, without clarifying the intent, the transition away from chemotherapy. This use may contribute to the persistent and pervasive sense that palliative care is equivalent to EOL care. However, palliative care is a specialized approach to the care of patients with serious or life-threatening illnesses; it is guided by principles and accomplished via a specific—but by no means exclusive—skill set distinct from the skills required to prescribe and manage chemotherapy. Hospice Hospice is a team-oriented approach to providing expert medical care, pain management, and emotional and spiritual support for patients whose life expectancy is 6 months or less. For more information, see Hospice. References: Several strategies can potentially improve the quality of oncologist-patient communication and decision making and facilitate the transition to end-of-life (EOL) care for patients who have advanced cancer. For more information, see the Education and Training in Cancer Communication section in Communication in Cancer Care. This section summarizes information relevant to the following strategies: Improving Prognostication A comprehensive review of various strategies to improve prognostication is beyond the scope of this summary. One study has recently published a concise overview.[1] There are several sources of prognostic information: Other investigators reported on the prognostic impact of a single self-reported QOL item in a cohort of almost 2,500 patients with newly diagnosed lung cancer.[3] Patients with a clinically significant QOL deficit had a median survival of 1.6 years, compared with 5.6 years without a deficit. Older age, worse performance status, and advanced stage of disease were also significant predictors; the QOL measure maintained independent influence on multivariable analysis. Novel Strategies to Improve Communication of Prognosis An Australian study demonstrated that most patients with metastatic cancer wanted information about life expectancy or prognosis; some patients thought that receiving estimates of worst-, typical-, and best-case outcomes would be acceptable.[4] Other investigators proposed that percentiles derived from multiple published OS curves for metastatic cancer would serve as the basis for estimating best- and worst-case scenarios.[5] Their subsequent work has established the following: Advance Care Planning (ACP) The 1990 Patient Self-Determination Act guarantees patients the right to accept or refuse treatment and to complete advance medical directives to document their EOL preferences or to appoint someone to make decisions on their behalf in the event that their ability to make decisions is compromised. Studies indicate, however, that many patients have not participated in effective ACP. Several reasons might explain the underuse of ACP:[10,11,12] This section provides a glossary of ACP terms and highlights the ambivalence that surrounds ACP. It includes a review of subsequent evidence that demonstrates the benefit of ACP, suggesting that a more nuanced approach to ACP will improve care near the EOL for patients who have advanced cancer. The language of ACP A brief glossary of the language of ACP may minimize confusion. A proxy can be chosen in addition to, or instead of, a living will. A durable power of attorney for health care allows the patient to be more specific about medical treatment than does a living will. Documenting ACP decisions Additional documents to communicate the patient's preferences include the following: Patient and physician ambivalence about ACP One of the major impediments to effective ACP is patient and physician ambivalence. Patients with advanced cancer may be ambivalent about engaging in ACP with their oncologists because they perceive that oncologists are reluctant to engage in this discussion.[11,13] For example, one group of researchers reported that 87% of women with metastatic breast cancer had discussed EOL decisions with family or friends; 75% had gathered information about advance directives; and 66% had a written advance directive.[14] However, only 19% of those women had discussed EOL decisions with providers; only 24% had shared the written advance directives with the providers; and only 14 % of providers were aware of the advance directives. A study of hospitalized elderly patients similarly found that many had thought about EOL preferences (76.3%) or had an advance care plan (47.9%), but relatively few (30.3%) had discussed them with their physicians.[15] These results suggest that patients engage in ACP but are reluctant to share their thoughts with their oncologists. Nevertheless, the oncologist is in the best position to know when to bring up the subject of EOL care, so he or she can initiate and thoughtfully guide the ACP discussion.[16] ACP Reconsidered Equivocal evidence is a source of physician ambivalence about ACP. Despite early concerns, however, there is growing evidence that ACP improves EOL care in patients with advanced cancer. Early concerns about limits of ACP Initial evidence about ACP was not favorable. For example, several studies found that: Potential benefits of ACP Since the 1990s, randomized trials of ACP have demonstrated some benefit. For example, a randomized study compared usual care with a structured approach that included a motivational conversation with a social worker, and a booklet that described ACP and sought information about the patient's desired health states and values.[22] The primary outcomes were comparisons of how closely providers and patients agreed about the following: The intervention was associated with increased frequency of ACP discussions between physician and patient (64% vs. 38%; P < .001), and the intervention group had higher levels of agreement for all three outcomes.[22] The effect of ACP on health care outcomes was not studied. A 2010 study monitored 309 patients older than 80 years and their caregivers for 6 months or until a patient died.[23] Researchers found that patients who engaged in ACP were more likely to have preferences that are known to others, and to receive care consistent with those preferences. In addition, compared with family members of patients in the control group, family members of patients in the intervention (with ACP) group who died had significantly less stress (intervention 5, control 15; P < .001), anxiety (intervention 0, control 3; P = .02), and depression (intervention 0, control 5; P = .002). Also, patient and family satisfaction were higher in the intervention group.[23] Studies funded by the Agency for Healthcare Research and Quality show that satisfaction in patients aged 65 years and older increased when patients engaged in ACP with their doctors. Specifically, patients who discussed their preferences for EOL care:[24] Surrogates experienced similar benefits.[24] A 2011 report also suggests that ACP contributes significantly to higher-value EOL care. Researchers demonstrated that the association between evidence of ACP and reduced EOL Medicare expenditures varied according to the level of spending on aggressive treatments near the EOL.[25] Treatment-limiting advance directives were associated with lower levels of Medicare spending, lower likelihood of in-hospital death, and increased use of hospice in high-spending regions but not in low- or medium-spending regions. At a minimum, the study suggests that ACP may be one explanation for lower-cost regions. A more nuanced approach to ACP Advocates maintain that ACP has been too narrowly construed as completion of a document that expresses a patient's EOL preferences or identifies a surrogate decision maker if a patient can no longer make decisions. Generally speaking, most patients from Western countries express greater needs for information related to their illnesses, treatment options, prognoses, future symptoms, likely trajectories of illness, and the dying process. Younger and more educated patients tend to seek more detailed information, while older patients and those from non-Western cultures tend to prefer nondisclosure. From that perspective, the limitations of ACP can be addressed by reconceptualizing ACP. One proposal is to imagine ACP as an ongoing discussion designed to prepare patients and their families or friends to participate more meaningfully in future medical decisions, as required.[26] A focus group study of patients and surrogate decision makers identified four salient themes in discussing future health care decisions:[27] Before discussing ACP, it is essential that the oncologist determine the information needs of the patient so that communication and ACP can be tailored accordingly.[28] Interventions to Support Patient-Oncologist Communication Various interventions to support patient-oncologist communication have been studied. There are few comparative trials, and the outcomes of interest have varied, so it is not possible to evaluate the interventions. However, the variety of interventions will allow the interested oncology clinician to find one of interest. Written information A myriad of informational booklets are available for patients to consult; prognostic information is discussed in some of them. Researchers conducted a mixed-methods study of the views of cancer patients on written materials provided by their oncologists.[29] Patients expressed concerns that conflicting information could undermine their trust in their oncologists, and this concern influenced their willingness to consult written materials. Another caution was raised by a randomized study that compared QOL and satisfaction between lung cancer patients scheduled for surgery who received verbal information only and those who received verbal and written information.[30] There were no differences in patient satisfaction with information; however, patients who received verbal and written information were less satisfied with the hospital stay. Thus, the presentation of written information may have unintended consequences, and oncologists may consider distributing only information that reflects their actual practices. Audiotaped consultations The impact of simply recording consultations on audiotape is significant, especially given the ease of doing so. In one randomized trial of initial consultations for all stages of cancer, 105 patients received audiotapes and 96 did not.[31] Patients who received the audiotapes were more satisfied and had better recall of information than did those who did not receive the tapes. In addition, younger patients were more satisfied, and older patients experienced enhanced recall. Another study reported similar results.[32] Video-recorded information The use of videos to facilitate discussions between providers, patients, and families and to assist with informed decision making has been effective in several randomized controlled trials.[33,34] In these studies, patients and caregivers (where appropriate) found the videos acceptable and were comfortable with their content. In the first study, 50 patients with malignant glioma were randomly assigned to hear a verbal narrative or to hear the same verbal narrative and be shown a video that described three types of care:[33] Participants in the sample had a mean age of 54 years; 44% were female, 50% did not want CPR at baseline, and 76% had advance directives. When asked postintervention about their preferences for type of care, 26% of participants in the verbal control group preferred life-prolonging care, compared with none in the video intervention group. More patients in the video intervention group preferred comfort care than did patients in the verbal control group (91% vs. 22%, respectively).[33] In the second study, 150 patients with advanced cancer from four cancer centers were randomly assigned to listen to a verbal description of CPR or to listen to the same verbal description and then be shown a 3-minute video of simulated CPR being done on a patient on a ventilator.[34] The primary outcome was participants' preference for or against CPR measured immediately after exposure to either modality. As in the first study, more patients receiving the video intervention with the narrative decided against CPR than did those receiving only the narrative (79% vs. 51%, respectively). Knowledge scores were also significantly higher in the video arm (P < .001). Results at the 6- to 8-week follow-up demonstrated fairly stable decisions. In a third single-center, randomized control trial, investigators assigned 150 hospitalized patients with advanced cancer and 44 of their caregivers to one of two groups. One group watched a 6-minute video about hospice, while the other was read an identical verbal description of hospice. The primary outcome, patient preference for hospice, did not significantly change and did not differ between the two groups postintervention (though both groups expressed high levels of preference for hospice at baseline). However, patients who watched the video had improved knowledge of hospice and were less likely to agree with the statement, "I think that hospice care is only about death." The intervention's most interesting impact, however, was on hospice use and length of stay. Among patients who died during the study, those who had been in the video group were more likely to have used hospice care (85.2% vs. 63.6%; P = .01) and had a longer hospice length of stay (median, 12 days vs. 3 days; P = .01). Caregivers in the video group were more likely to prefer hospice and had improved hospice knowledge, though findings were limited by the small number of caregivers in the study.[35] These studies suggest that videos can be useful for improving patient knowledge related to ACP and may even affect patients' ACP and end-of-life decision making. The results are encouraging regarding the value of videos to supplement oncologist-patient communication and education. Question prompt lists Patients may have difficulty posing questions about sensitive and difficult issues such as prognosis or EOL care. Prompt lists can help guide patients through such questions. Researchers developed and tested a question prompt list for advanced cancer patients to use during consultation with a palliative care physician.[36] Patients randomly assigned to the intervention arm more frequently asked more questions related to prognosis and EOL issues than did patients in the control group (30% vs. 10%; P = .001). There were no negative effects on patient satisfaction or anxiety. A slightly different strategy—addressing the prompt list to informal caregivers of patients with advanced cancer—was evaluated in another study and appeared promising.[37] Cancer consultation preparation package (CCPP) A CCPP developed by one group of researchers consists of four components:[38] A randomized trial of the CCPP versus a control booklet in 164 initial consultations for patients with a variety of cancers at different stages demonstrated that patients receiving the CCPP asked significantly more questions (11 questions vs. 7 questions; P = .005) without increasing their anxiety. However, patients assigned to the CCPP did not participate in decision making more often or more actively than did patients in the control group.[38] Pamphlet and psychologist-facilitated discussion In a randomized trial, an ACP pamphlet supplemented by a discussion with a psychologist was compared with usual care.[39] Patients in both cohorts had equivalent rates of DNR orders (68% vs. 76%), but DNR orders for the intervention group were placed at a median of 27 days, compared with 12.5 days for the control group (P = .03). Intervention patients were less likely to die in the hospital (19% vs. 50%), and there was no increased sense of anxiety. Interpretation of the study results is hampered by a high rate of loss to follow-up, and the intention-to-treat analysis was not significant. Decision Aids Decision aids are more complicated interventions designed to provide patients with a balanced summary of information about potential treatment options and possible outcomes, allowing patients to make informed decisions consistent with their preferences. Decision aids complement the patient-oncologist relationship and have been studied in two situations relevant to the care of patients with advanced cancer: the decision to undergo chemotherapy in advanced cancer and the decision to prepare for the EOL. The decision to undergo chemotherapy In a 2011 study of patients with metastatic colorectal cancer who were deciding whether to initiate first-line systemic chemotherapy, 207 patients were randomly assigned to receive either a standard oncology consultation or a standard consultation supplemented by a take-home booklet and audio recording of the consultation.[40] Patients assigned to the decision aid demonstrated increased understanding of the prognosis, treatment options, and goals of treatment. There were no differences in decisional conflict, levels of anxiety, patient achievement of preferred level of involvement in decision making, or treatment selected. The decision to engage in ACP In a pilot semirandomized trial, patients with advanced cancer who met with a trained care planning mediator were more likely to discuss future preferences with families and friends (but not with their oncologists).[41] Slightly more than half of patients with advanced cancer were willing to meet with an independent trained counselor about ACP. Patients assigned to meet with the mediator tended to be less satisfied with communication with health care professionals and less satisfied with future care. Thus, while ACP with a trained mediator is feasible, the costs of additional personnel may be prohibitive and create unmet expectations in patients. In a Korean randomized controlled trial, the impact of decision aids on preferences for active treatment, life-prolonging treatment, or hospice care was examined, and knowledge about ACP and cardiopulmonary resuscitation (CPR) was also assessed: 204 patients with advanced cancer were assigned to either a group that received a video and workbook training on ACP (intervention group) or another group that received a video and workbook training on managing cancer pain (control group).[42] Seven weeks post intervention, patients in the intervention group who assumed a prognosis of expected death within several weeks or several months expressed a greater preference for hospice care than patients in the control group. Patients in the intervention group also had greater knowledge about CPR postintervention. There were no statistically significant differences between the two groups with respect to patients documenting advance care preferences or discussing EOL care with their family, friends, or physicians. Decisions related to resuscitation status Several approaches to facilitate discussion about a patient's resuscitation preferences and status have been studied. The approaches include the following: In several studies, DNR orders were more common in the intervention group or occurred earlier. The secondary outcomes included earlier and higher rates of discussions with patients and family members regarding resuscitation, lower decisional conflict and uncertainty, and more value clarification. In at least two studies, there was no increase in anxiety and/or depressive symptoms. These decision aids are limited to use among adult patients. Physician attitudes toward decision aids Researchers conducted a cross-sectional survey of Canadian medical, radiation, and surgical oncologists to determine their levels of awareness and utilization of patient decision aids.[45] Slightly fewer than half (46%) were aware of decision aids relevant to their practices, and only 24% used aids in their practices. Most respondents recognized the important clinical outcomes associated with decision aids, i.e., increased knowledge, reduced anxiety, and increased patient satisfaction. Lack of awareness was the most frequently cited barrier to use (69%), followed by lack of resources (24%) and lack of time to learn about decision aids (24%). Only 3% of respondents cited lack of time as a barrier to the use of decision aids. Strategies to introduce decision aids into clinical practice are clearly needed and could start with educating oncologists about available decision aids. Integrating Palliative Care and Conventional Cancer Care In 2016, the American Society of Clinical Oncology published an updated guideline advising its membership that "inpatients and outpatients with advanced cancer should receive dedicated palliative care services, early in the disease course, concurrent with active treatment."[46] The authors primarily based their recommendation on two randomized clinical trials of palliative care interventions during conventional cancer care for people with metastatic cancer that supported results of several older and inconclusive randomized clinical trials.[47] Subsequently, two additional randomized trials comparing outcomes between cohorts of patients who received conventional oncology care and a concurrent palliative care intervention or conventional oncology care at different times have been reported.[48,49] The four studies are summarized below: ENABLE II: A randomized trial of an advance practice nurse–led psychoeducational intervention This group of investigators reported the results of Project ENABLE (Educate, Nurture, Advise Before Life Ends).[50] Three hundred twenty-two patients with advanced cancer were randomly assigned to receive either usual care or a multicomponent psychoeducational intervention conducted by advance practice nurses concurrent with usual care. The primary outcomes were QOL, as measured by the Functional Assessment of Chronic Illness Therapy for Palliative Care; symptom intensity, as measured by the Edmonton Symptom Assessment Scale; and mood, as measured by the Center for Epidemiological Studies Depression Scale. The intervention was based on the chronic care model; the goal was to encourage patient involvement in care planning, self-management, and empowerment. The intervention consisted of four weekly educational sessions and a monthly follow-up. The four modules consisted of the following: The mean scores for the participants in the intervention group were 4.6 higher for QOL (P = .02); 27.8 lower for symptom intensity (P = .06); and 1.8 lower for depressed mood (P = .02). Patients who died during the study had less-marked treatment effects. There was no difference in OS or use of chemotherapy or aggressive medical care near the EOL. Furthermore, there were no differences between groups in rates of referral to palliative care or hospice.[50] Randomized trial of concurrent palliative care and standard oncology care for patients with metastatic lung cancer The second study randomly assigned 151 patients with newly diagnosed metastatic NSCLC to receive either standard oncology care or standard oncology care with the early integration of palliative care. Patients assigned to palliative care met with a member of the palliative care team within 3 weeks of diagnosis. General guidelines called for palliative care clinicians to pay specific attention to the following: The primary and secondary analyses of the study have been reported in an original publication and then in several secondary publications,[51,52,53] summarized below. Patients in the palliative care cohort who had an accurate understanding of prognosis were less likely to receive chemotherapy than were patients who had an inaccurate understanding of prognosis (9.4% vs. 50%; P = .02). A similar effect was not seen in patients in the standard care arm; the percentage of patients in the standard care arm receiving chemotherapy did not differ by perception of prognosis. Cluster-randomized trial of early palliative care for patients with advanced cancer This single-blinded study reported the outcomes of patients who were assigned to receive palliative care and conventional cancer care or conventional cancer care alone.[57] The palliative care intervention included an initial visit followed by monthly palliative care clinic visits, with telephone follow-up and direct access to the inpatient palliative care unit as needed. The primary outcome was the change score for the Functional Assessment of Chronic Illness Therapy—Spiritual Well-Being (FACIT-Sp) at 3 months. Secondary outcomes were the FACIT-Sp at 4 months and other validated measures of QOL, symptom burden, satisfaction with care, and quality of medical interactions at 3 and 4 months. There were no statistically significant differences in the change FACIT-Sp scores at 3 months, but differences did emerge at 4 months; in addition, there were significant differences in the secondary outcomes at 3 and 4 months. These results confirm the benefit of concurrent palliative care and suggest that the benefits of palliative care will increase over time. The coordination of care between the Princess Margaret Cancer Centre and Community Care Access Centres may make the findings difficult to replicate.[57] ENABLE III: Early versus delayed initiation of concurrent palliative oncology care This study compared the outcomes of 207 patients randomly assigned to begin receiving palliative care interventions within 30 to 60 days of diagnosis versus 3 months from diagnosis of advanced cancer.[49] The weekly telephone coaching sessions described in the summary of ENABLE II included a three-session life review component and was designed to specifically intervene with caregivers. The outcomes of interest were QOL, symptom impact, mood, resource use, and improved 1-year survival. Early palliative oncology care was associated with improved survival (63% vs. 48%, P = .038). There were, however, no statistically significant differences in patient-reported outcomes or resource use. Furthermore, the OS log-rank test did not reveal a significant difference, suggesting the survival curves converge after the 1-year point. A few limitations of the study potentially complicate interpretation. First, the study did not meet its accrual goal of 360 patients, in part because of high rates of ineligibility after screening and patient refusal. Second, the intention-to-treat analysis may have missed differences due to variations in the "amount" of the intervention completed. Third, the multiple outcomes may have limited the statistical power to detect meaningful differences, especially given the smaller-than-desired sample size. Thus, a more-accurate interpretation of the results may be that the optimal time of palliative care interventions remains undetermined. In contrast to the results for patients, the ENABLE III intervention improved outcomes for caregivers.[58] A total of 122 caregivers were randomly assigned to receive, either early or at a later time, three weekly telephone coaching sessions, monthly follow-up, and a bereavement call. For all caregivers, the early intervention was associated with lower depression scores at 3 months. Caregivers of decedents who received palliative care early had lower depression and burden in the terminal phase. These results are notable in establishing a benefit to caregivers. Challenges to the early integration of palliative care Several of the more relevant challenges to the integration of palliative care into standard oncology care are listed below, followed by a summary of the available data. One potential solution to the workforce shortage is to train oncologists in the skills of so-called primary palliative care.[60] The skills of primary palliative care include basic discussions about prognosis, goals of treatment, suffering, or code status and symptom management, thus reserving specialty referrals for assistance with conflict resolution regarding goals or methods of treatment. References: Perspective In 1961, a survey of 219 physicians was published, with 88% of respondents indicating that their "usual policy about telling patients" they have cancer was "don't tell"; however, 34% of the group indicated that they occasionally made exceptions and disclosed the diagnosis, most often to family members.[1] The identical survey was repeated in 1977, with a different group of 264 physicians; at that time, 98% of respondents indicated that telling a patient about the diagnosis of cancer was their usual policy, although 28% reported occasionally making an exception.[2] Despite this progress, the effective communication of prognosis remains a challenge, as measured by patient-reported estimates of life expectancy or goals of treatment. For example, a 1984 study reported that 37% of patients with metastatic, incurable cancer felt that treatment would cure their disease.[3] Similarly, another study reported in 2012 that 69% of patients with advanced lung cancer and 81% of patients with advanced colorectal cancer enrolled in the Cancer Care Outcomes Research and Surveillance (CanCORS) study were unaware that chemotherapy was not likely to cure their disease.[4] In fact, almost 40% of patients with colorectal cancer responded that chemotherapy was very likely to cure the disease. A related analysis of the CanCORS data set revealed that only 16.5% of patients reported an accurate awareness of their prognosis.[5] Similar results have been reported from a study of patients with advanced cancer that assessed their understanding of their prognosis at enrollment and 12 weeks later.[6] Potential explanations for the communication deficits regarding prognosis are summarized in the Potential Barriers to Planning the Transition to End-of-Life Care section. Regardless of the explanation, however, misunderstandings about prognosis compromise a patient's ability to make informed health care decisions consistent with his or her values, goals, and preferences. Ultimately, each oncology clinician chooses, on some level, when and how to explain to a patient with advanced cancer that disease-directed treatments are unlikely to prove effective and a continued focus on treatment risks harming the patient. The goal of this summary section is to provide clinicians with frameworks for considering their role in planning the transition to end-of-life (EOL) care and discussing the concept of transition with patients and their loved ones. The Patient-Clinician Relationship and Planning the Transition to EOL Care It is important for oncology clinicians to consider how their relationship with patients will help patients express preferences that are consistent with their values and goals for receiving high-quality EOL care. One study proposed the following four models of the physician-patient relationship, based on the goals of the interaction, the physician's obligations, the role of patient values, and how patient autonomy influences the relationship:[7] In a carefully reasoned argument, the authors concluded that the preferred choice is the deliberative model, which can be paraphrased as a belief that patients' goals and preferences are open to development and evolution. The goal of this relationship is to help the patient choose the best health-related goals and options that can be achieved, given the clinical situation. The physician's obligation is to articulate for the patient the most compelling goals and preferences and to inform the patient of other options. In that sense, the physician is a teacher.[7] Qualitative studies of patients' perception of decision making suggest that the deliberative clinician-patient relationship is especially critical in advanced-stage cancer. For example, one group of investigators interviewed 36 patients with potentially curable esophageal cancer about their decision to undergo esophagectomy. The investigators anticipated that themes related to autonomy, shared decision making, and information disclosure would become apparent; however, the following themes emerged:[8] Another group of investigators interviewed patients with pancreatic carcinoma and identified a change in attitudes toward treatment decision making over time.[9] Initially, patients described little interest in details about medical or surgical treatments and emphasized their trust in the physician. Later, as patients had more experience with disease progression and treatment, they described seeking a more proactive role in treatment decisions. All patients reported discussions about their poor prognosis, but a common theme was the difficulty in recognizing when it was time to stop treatment and transition to EOL care. Planning the Transition From Diagnosis to EOL Care The word transition implies a passage from one place to another. Planning the transition to EOL care, therefore, requires a shared understanding of where the patient is in the advanced-cancer trajectory and why a transition is necessary or advisable. The appropriate time to transition to EOL care is when the change is most consistent with the patient's goals of care. In determining when to transition to EOL care, the oncology clinician strives to be a teacher and trusted friend. The clinician must present his or her opinion of the effectiveness of continued cancer-directed treatments and help the patient understand the patient's own values, perspectives, goals of care, and preferences. In this way, the clinician aids the patient in constructing treatment and care preferences toward the EOL.[10] The concept of transition is often invoked from the health care provider's perspective.[11,12] For example, patients who received adjuvant chemotherapy with curative intent are often spoken of as making a transition from the curative to the palliative phase of treatment. The figure below depicts a phase model of planning the transition to EOL care for patients with advanced cancer. Panel A of the figure demonstrates the increasingly unfavorable risk-benefit ratio of disease-directed treatments over time. This conceptualization is also from the oncology clinician's perspective. The oncology clinician could choose two tactics to develop a shared understanding of the decline in the risk-to-benefit ratio: Panel B of the figure represents the concept of transitions. The disease trajectory is divided into five relatively discrete phases, from the diagnosis of advanced cancer to death. The first two phases are dominated by disease-directed strategies, including a phase in which the intent of disease-directed treatment is cure and a second phase in which the intent of treatment is symptom relief, disease control, and, perhaps, improved life expectancy. The EOL phase is subdivided into a time in which the patient is not receiving therapy but may still anticipate treatment in the future, and a phase in which the goal of care is to ensure that the EOL is free from undue burden or distress. Similar figures are often modified to include a diagonal line depicting a gradual increase in the focus on palliative care as the cancer progresses and life is more likely to end. The figure represents the advanced-cancer trajectory to help oncologists formulate and articulate the potentially salient differences between the phases from the oncologist's perspective in terms of the following: The oncology clinician should then endeavor to communicate the information in a compassionate manner that is comprehensible to the patient and family. For more information, see the Strategies to Improve Patient-Oncologist Communication and Decision Making in Advanced Cancer section. Clearly describing each phase, however, does not necessarily inform patients of when it is time to transition. A focus group study of health care providers, patients with advanced cancer and other terminal illnesses, and family members prioritized three discrete communication skills as valuable to the dying person:[16] In conclusion, the right time to transition from disease-directed treatments toward EOL care depends, in large part, on the patient's goals and preferences. The evidence indicates that conversations about EOL care are difficult but of great benefit to the patient. Oncologists who delay initiating these important conversations or who communicate in ambiguous or difficult-to-understand language fail to meet "the physician's central task in caring for a gravely ill person near death[, which] is to accompany and guide the patient, who as a rule does not want to be dead, through the critical transition."[17] References: The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above. Updated statistics with estimated new deaths for 2025 (cited American Cancer Society as reference 1). Factors That Influence End-of-Life Care Decisions and Outcomes Updated American Cancer Society as reference 7. This summary is written and maintained by the PDQ Supportive and Palliative Care Editorial Board, which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® Cancer Information for Health Professionals pages. Purpose of This Summary This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about planning for end-of-life care in advanced cancer. It is intended as a resource to inform and assist clinicians in the care of their patients. It does not provide formal guidelines or recommendations for making health care decisions. Reviewers and Updates This summary is reviewed regularly and updated as necessary by the PDQ Supportive and Palliative Care Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH). Board members review recently published articles each month to determine whether an article should: Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary. The lead reviewers for Planning the Transition to End-of-Life Care in Advanced Cancer are: Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's Email Us. Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries. Levels of Evidence Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Supportive and Palliative Care Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations. Permission to Use This Summary PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as "NCI's PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary]." The preferred citation for this PDQ summary is: PDQ® Supportive and Palliative Care Editorial Board. PDQ Planning the Transition to End-of-Life Care in Advanced Cancer. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/about-cancer/advanced-cancer/planning/end-of-life-hp-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389513] Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online, a collection of over 2,000 scientific images. Disclaimer The information in these summaries should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page. Contact Us More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website's Email Us. Last Revised: 2025-02-12 This information does not replace the advice of a doctor. Ignite Healthwise, LLC disclaims any warranty or liability for your use of this information. Your use of this information means that you agree to the Terms of Use and Privacy Policy. Learn how we develop our content. Healthwise, Healthwise for every health decision, and the Healthwise logo are trademarks of Ignite Healthwise, LLC.Topic Contents
Planning the Transition to End-of-Life Care in Advanced Cancer (PDQ®): Supportive care - Health Professional Information [NCI]
Overview
Quality of End-of-Life Care in Patients With Advanced Cancer
Factors That Influence End-of-Life Care Decisions and Outcomes
Potential Barriers to Planning the Transition to End-of-Life Care
Supportive Care, Palliative Care, and Hospice in Advanced Cancer
Strategies to Improve Patient-Oncologist Communication and Decision Making in Advanced Cancer
Author/Reference Recruitment (No.) Selected Eligibility Criteria Randomized Potentially Eligible Screened Type of Cancer Time from Diagnosis Oncologist-Estimated Survival GI = gastrointestinal; GU = genitourinary, hem = hematologic. Bakitas et al., 2009[50] 322 681 1,222 GI; lung; GU; breast Within 8–12 weeks ~1 year Temel et al., 2010[51] 151 Lung ≤8 weeks Not specified Zimmermann et al., 2014[48] 461 892 3,293 GI; lung; GU; breast; gynecologic Not specified 6–24 months Bakitas et al., 2015[49] 207 545 1,468 GI; lung; GU; breast; other; hem malignancy Early: 30–60 days; delayed: >90 days 6–24 months End Point Measure CARES-MIS = Cancer Rehabilitation Evaluation System Medical Interaction Subscale; CES-D = Center for Epidemiologic Studies Depression Scale; ED = emergency department; ENABLE = Educate, Nurture, Advise Before Life Ends; ESAS = Edmonton Symptom Assessment System; FACIT-Pal = Functional Assessment of Chronic Illness Therapy - Palliative Care; FACIT-Sp = Functional Assessment of Chronic Illness Therapy - Spiritual Well-Being; FACT-L = Functional Assessment of Cancer Therapy - Lung; FAMCARE-P16 = 16-item measure of patient satisfaction with cancer care; HADS = Hospital Anxiety and Depression Scale; ICU = intensive care unit; NR = outcome measured but not reported; QUAL-E = Quality of Life at the End of Life; — = outcome not measured. Bakitas et al., 2009 (ENABLE II)[50] Temel et al., 2010[51] Zimmermann et al., 2014[48] Bakitas et al., 2015 (ENABLE III)[49] Quality of life FACIT-Pal FACT-L FACIT-Sp; QUAL-E FACIT-Pal; QUAL-E Mood CES-D HADS — — Symptoms ESAS — QUAL-E ESAS Satisfaction with care — — FAMCARE-P16 — Medical interactions — — CARES-MIS — Chemotherapy use NR NR NR NR End-of-life resource use Hospital/ICU days; ED visits Hospital/ICU days; ED visits; chemotherapy within 14 days of death; hospice utilization; place of death Not specified Hospital/ICU days; ED visits; chemotherapy within 14 days of death; place of death Median overall survival Yes Yes — Yes Outcome Author/Reference ENABLE = Educate, Nurture, Advise Before Life Ends; NR = outcome measured but not reported; NS = no significant difference in outcomes detected; PC = favors palliative care intervention; — = outcome not measured. Bakitas et al., 2009 (ENABLE II)[50] Temel et al., 2010[51] Zimmermann et al., 2014[48] Bakitas et al., 2015 (ENABLE III)[49] Quality of life PC PC NS at 3 months; PC at 4 months NS Mood PC PC NS at 3 months; PC at 4 months NS Symptoms NS — NS at 3 months; PC at 4 months NS Satisfaction with care — — PC at 3 months; PC at 4 months — Medical interactions — — NS at 3 months; NS at 4 months — End-of-life resource use NS NR NR NS Overall survival NS PC NR Early The Oncology Clinician's Role in Planning the Transition to End-of-Life Care
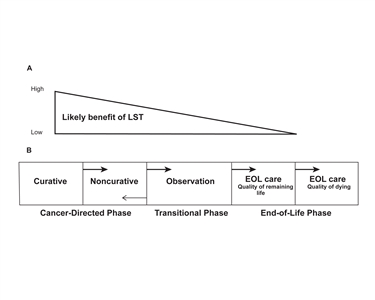
A phase model of planning the transition to end-of-life (EOL) care in advanced cancer. The trajectory of advanced cancer begins with the realization that disease-directed treatments are no longer curative. Over time, the patient, family, and oncology clinician should strive to develop a shared understanding of available treatments and recognize when the likelihood of benefit decreases in relation to the potential harm of treatment. LST = life-sustaining treatment; EOL = end-of-lifeLatest Updates to This Summary (02 / 12 / 2025)
About This PDQ Summary
Our Health Library information does not replace the advice of a doctor. Please be advised that this information is made available to assist our patients to learn more about their health. Our providers may not see and/or treat all topics found herein.Planning the Transition to End-of-Life Care in Advanced Cancer (PDQ®): Supportive care - Health Professional Information [NCI]



