Our Health Library information does not replace the advice of a doctor. Please be advised that this information is made available to assist our patients to learn more about their health. Our providers may not see and/or treat all topics found herein. This information is produced and provided by the National Cancer Institute (NCI). The information in this topic may have changed since it was written. For the most current information, contact the National Cancer Institute via the Internet web site at http://cancer.gov or call 1-800-4-CANCER. Gastric cancer is one of the most commonly diagnosed cancers in the world, with an incidence of over 1 million cases and an estimated 769,000 deaths occurring in 2020. Globally, this cancer ranked fifth for cancer incidence and fourth for cancer mortality in 2020.[1,2] In 2024, there will be an estimated 26,890 new cases of gastric cancer and 10,880 deaths from gastric cancer in the United States.[3] In clinic-based populations, approximately 15% to 20% of patients with gastric cancer are found to have pathogenic variants in hereditary cancer genes.[4,5] The genes that can predispose individuals to hereditary diffuse gastric cancer (HDGC) include the following: HDGC is the most common hereditary cancer syndrome that can predispose individuals to gastric cancer. This disease does not have an easily detectable precursor lesion. For more information, see Hereditary Diffuse Gastric Cancer. There are also hereditary cancer syndromes that can cause other forms of gastric cancer. These syndromes include the following: In each of these syndromes, gastric polyps and gastric cancer risk are of secondary importance to colorectal polyps and colorectal cancer. Gastric polyp burden (including polyp count and size) and gastric cancer risk may vary between individuals. For more information on these genetic syndromes, see Table 1. Gastric Cancer Risk Factors Environmental risk factors Incidence rates for gastric cancer are the highest in Eastern Asia and Eastern Europe, while the rates in Northern America, Northern Europe, and Africa are generally low.[2] These regional differences are most likely affected by environmental and hereditary risk factors. Environmental risk factors for gastric cancer include the following: 1. Helicobacter pylori (H. pylori) infection. 2. Diet. 3. Migrant effects. 6. Emerging evidence for obesity and gastroesophageal reflux disease (GERD). 7. Racial and ethnic disparities. Intensive surveillance may be clinically warranted in individuals with hereditary, environmental, or ethnicity-based gastric cancer risk factors. However, more data are needed to show whether these risk factors increase or decrease gastric cancer risk in individuals with hereditary gastric cancer pathogenic variants. For more information about risk factors for gastric cancer in the general population, see Stomach (Gastric) Cancer Screening. Familial risk factors Individuals with family histories of gastric cancer have an increased risk of developing gastric cancer.[26] For example, when individuals had a first-degree relative with gastric cancer, their RRs to develop gastric cancer ranged from 1.5-fold to 3.5-fold in seven different studies.[27,28,29,30,31,32,33] However, these were retrospective studies, and risk estimates may have been confounded by recall bias (i.e., recalling cancers in relatives), shared environmental factors, shared dietary factors, and different countries that studies were conducted in. The most notable environmental/dietary risk factor was H. pylori infection. When an individual has an H. pylori infection, other people in this individual's family also have an increased chance of developing an H. pylori infection. Smaller cohort studies have suggested the presence of a residual heritable risk for gastric cancer after correcting for the presence of H. pylori. Most of these studies did not account for hereditary gastric cancer pathogenic variants.[34] A large retrospective review of Japanese individuals (both with and without gastric cancer) found a strong, additive risk for gastric cancer in individuals with H. pylori infection, especially in those with ATM, BRCA1, BRCA2, or PALB2 pathogenic variants.[11] A Scandinavian study demonstrated that the concordance value for gastric cancer was higher in monozygotic twins than it was in dizygotic twins. This suggests that gastric cancer risk may be heritable.[35] Families with multiple gastric cancer cases have also been used to study the heritability of gastric cancer. A report in 1964 described a large, native Maori family from New Zealand.[36] Then, in the late 1990s, another study established genetic linkage between CDH1, a gene that codes for a cell-adhesion molecule, and gastric cancer cases from the original Maori family (identified in 1964) and two additional Maori families. In this study, researchers found that CDH1 was downregulated in Maori individuals with advanced gastric cancer.[36,37,38,39]Germline CDH1 pathogenic variants were first found in affected individuals in these Maori families, and later in families around the world with hereditary diffuse gastric cancer. Subsequently, female lobular breast cancer and orofacial clefts were added to the phenotypic spectrum of CDH1 pathogenic variants.[40] Precancerous Lesions In the stomach, gastric adenocarcinoma can arise from visible lesions, from the histological progression of mucosal atrophy or, less commonly, from invisible lesions (i.e., submucosal infiltration, as seen in diffuse-type gastric adenocarcinoma). This section reviews both gastric polyps and the histological changes associated with chronic gastritis that can predispose an individual to gastric cancer. Gastric polyps Gastric polyps are common in the U.S. population and are found in up to 6% of individuals who undergo endoscopy. However, these polyps rarely progress to become gastric cancer.[41] When gastric polyps are isolated, they usually are sporadic in nature. However, gastric polyps can also be associated with hereditary cancer syndromes, particularly the hamartomatous and polyposis syndromes. Gastric polyps can present with the following pathologies: In general, the malignant potential of a polyp is determined by the polyp's histology, size, and degree of dysplasia, if present. Adenomatous and hyperplastic polyps have the highest malignant potentials, especially when H. pylori is present. In contrast, fundic gland polyps (with or without dysplasia) rarely progress to become gastric cancer, even in individuals who have FAP.[43] Fundic gland polyps of the stomach are common and typically associated with long-term proton pump inhibitor (PPI) use.[44] Fundic glands polyps generally do not increase gastric cancer risk because these polyps are not neoplastic in nature. However, GAPPS is an exception to this rule. In GAPPS, gastric cancer risk is strongly correlated with fundic gland polyposis. For more information about GAPPS and FAP, see Genetics of Colorectal Cancer and Gastric Adenocarcinoma and Proximal Polyposis of the Stomach. Inflammatory and hyperplastic polyps can present with many different pathological features. Unlike the colon, in which hyperplastic polyps carry little or no cancer risk, hyperplastic polyps of the stomach can enlarge and develop dysplastic foci.[45] These types of polyps are not clearly associated with known hereditary gastric cancer syndromes. Hamartomatous polyps are seen in the stomachs of patients with PJS, JPS, and PHTS. For more information on these hereditary cancer syndromes, see Genetics of Colorectal Cancer. Hamartomatous polyps are typically not dysplastic in nature. However, they can develop dysplastic foci. Consequently, these polyps can be associated with an increased gastric cancer risk, especially in individuals with hereditary gastric cancer syndromes. It is unclear if hamartomatous gastric polyps eventually become gastric cancer or if they are merely indicative of a high-risk environment in the stomach. The risk of gastric cancer is estimated by the hereditary cancer syndrome. Gastric adenomas make up approximately 10% of all gastric polyps. A gastric adenoma's malignant potential is determined by its histological subtype and size.[46] For example, pyloric gland adenomas and intestinal-type gastric adenomas have the highest malignant potentials, while foveolar-type gastric adenomas have the lowest malignant potential.[47,48] Chronic gastritis Chronic gastritis, a histological diagnosis defined by the presence of a mononuclear cellular infiltrate in the lamina propria of the stomach, occurs due to environmental exposures or autoimmune gastritis. Chronic gastritis can lead to gastric intestinal metaplasia. H. pylori is the most common risk factor for intestinal metaplasia. Other risk factors for intestinal metaplasia include pernicious anemia, autoimmune gastritis, ethnicity, a family history of gastric cancer, dietary habits, smoking, and alcohol use.[49] After the development of chronic gastritis, the gastric mucosa may undergo a series of progressive changes, known as Correa's cascade, which can eventually result in gastric cancer. The steps of Correa's cascade include the following: Although these histological changes are associated with increased gastric cancer risk in the general population, the prevalence of findings and associated cancer risk in hereditary cancer syndromes is unknown. Each step in Correa's cascade only occurs in a minority of patients. There is considerable controversy regarding appropriate surveillance in patients with intestinal metaplasia.[52] Endoscopic assessment Gastric cancer screening is not routinely performed in the United States, where gastric cancer incidence is relatively low. Gastric cancer screening is also not usually performed in individuals with family histories of gastric cancer in which a hereditary gastric cancer pathogenic variant has not been identified. Large, prospective multicenter studies are needed to determine if gastric cancer screening programs would improve mortality in individuals from the United States with family histories of gastric cancer. In the United States, gastric cancer surveillance guidelines only exist for individuals with pathogenic variants in CDH1 or other gastric cancer risk genes (for more information, see Table 1).[53,54] The International Gastric Cancer Linkage Consortium recommends that individuals with family histories of diffuse gastric cancer without identifiable genetic causes (this is known as HDGC-like syndrome) participate in gastric cancer surveillance.[55] For more information, see the Definition and Management of Hereditary Diffuse Gastric Cancer (HDGC)–Like Families section in Hereditary Diffuse Gastric Cancer. When evaluating any patient with hereditary gastric cancer risk, endoscopy includes assessment for gastric polyps and H. pylori —despite a lack of evidence demonstrating that H. pylori increases gastric cancer risk in the setting of all hereditary cancer syndromes.[11] A small focus of intestinal metaplasia in the antrum is shown below. Intestinal metaplasia is not always visible on endoscopy. Instead, it is often found when taking random biopsies of the stomach.[49] Emerging research has identified measures to improve endoscopic detection of such foci. Populations with high gastric intestinal metaplasia and gastric dysplasia prevalence rates have high incidence rates of gastric cancer.[49] References: Approximately 15% to 20% of gastric cancers may be caused by hereditary gastric cancer syndromes.[1,2,3] When providers suspect that an individual has a hereditary gastric cancer syndrome, it is recommended that they obtain a detailed, three-generation family history and perform genetic testing, if appropriate. The collection of a detailed personal and family history is integral for gastric cancer risk assessment. The following items are important to elicit when collecting a patient's family history: a It is recommended that family history analysis focus on gastric cancer and other cancers (such as lobular breast cancer) that may be associated with hereditary diffuse gastric cancer (HDGC). For information on collecting a family history, see the Documenting the family history section in Cancer Genetics Risk Assessment and Counseling. In busy clinical settings, it might not be feasible to obtain a complete family history.[5] Studies have found that people often have incomplete or inaccurate information about the cancers that occurred in their relatives.[6,7,8,9] Furthermore, patients often over- or under-report the number of individuals in their families who have had gastric cancer. Many factors can contribute to inaccurate reporting of a gastric cancer family history. In medicine, the stomach has a distinct definition and anatomical location in the body. The public's definition of the stomach is more ambiguous, which could lead to reporting inaccuracies.[7] There are multiple organs inside the abdominal cavity that patients could confuse with the stomach. Studies have demonstrated that many patients do not know where organs are located in the body.[10] The poor validity of self-reported gastric cancer family histories needs to be considered during a patient's genetic evaluation and when creating an individual's clinical management recommendations. Studies have assessed the accuracy of self-reported family histories by comparing collected information with confirmation sources like death certificates, cancer registries, and histopathological records.[6,7,8,9,11] One retrospective study from the United Kingdom compared 595 clinical notes to confirmation sources. This study found increased error when patients reported malignancies located in the abdominal cavity.[7] A systematic review found that only 22% to 67% of reported stomach cancer diagnoses were verified by confirmation sources.[11] For more information, see the Accuracy of the family history section in Cancer Genetics Risk Assessment and Counseling. Obtaining pathological documentation of personal/family histories of cancer and polyps is also integral when assessing a patient's hereditary gastric cancer risk. This documentation is especially important because genetic testing criteria has shifted over the years and has, in turn, broadened clinical criteria.[12] It is important to obtain pathology reports from patients to confirm the histological subtypes of cancers when possible. Signet ring cell carcinoma, diffuse gastric cancer, and poorly differentiated carcinoma pathologies are characteristic of hereditary gastric cancer syndromes, particularly CDH1-associated HDGC. These histologies are commonly associated with linitis plastica (i.e., diffusely infiltrated, non-distensible stomach). Multiple gastrointestinal polyps (e.g., tubular adenomas, hamartomas) are associated with other hereditary cancer syndromes, such as Peutz-Jeghers syndrome, juvenile polyposis syndrome, familial adenomatous polyposis (FAP)/gastric adenocarcinoma and proximal polyposis of the stomach (GAPPS).[13] Fundic gland polyps are one of the most common findings on routine upper endoscopies. While these fundic gland polyps are a characteristic finding in individuals with FAP, they are also found often in individuals in the general population who take proton pump inhibitors. Hence, fundic gland polyps are not usually indicative of an underlying hereditary cancer syndrome. For more information about the cellular classification of gastric cancers, see Gastric Cancer Treatment. The diagnosis of cancers before age 50 years is a hallmark feature of hereditary cancer syndromes. Attenuated forms of hereditary cancer syndromes also exist and are increasingly recognized. In these attenuated syndromes, the ages of gastric cancer onset can occur after age 50 years.[14] Genetic Testing Approach Patients who develop gastric cancer usually present at an advanced stage.[15] This is important to consider when selecting a genetic testing approach. Genetic testing is time-sensitive for patients with advanced gastric cancer and should include informed consent, sample collection, and an explanation of genetic test results.[16] However, the timing of genetic testing will need to be balanced with the patient's competing treatment priorities and performance status. For more information, see the Value of testing an affected family member first section in Cancer Genetics Risk Assessment and Counseling. Germline genetic testing criteria exists for the following genes associated with gastric cancer and/or gastric polyps: CDH1, CTNNA1, APC, STK11, SMAD4, and the Lynch syndrome genes (MLH1, MSH2, MSH6, EPCAM).[12] One possible genetic testing approach is to first test for gene(s) in which clinical criteria for hereditary cancer syndrome(s) are met. This approach may not be ideal when a patient's family history includes cancers that can be associated with multiple hereditary gastric cancer syndromes. This can create a lengthy and expensive evaluation process if each suspected gene is tested sequentially after uninformative results. When the cancers in a family history overlap with multiple hereditary gastric cancer syndromes, providers can first test the gene(s) at the top of the differential diagnosis list. If pathogenic variants are not identified, providers can then reflex to a larger hereditary gastric cancer multigene panel. If this single-gene genetic testing approach is preferred, providers may need to identify a lab that offers the option to reflex to other genes before beginning the genetic testing process. When the patient has multiple cancers in the family that overlap with more than one hereditary cancer syndrome, another option is ordering multigene panel testing for all suspected genes. Multigene panel testing is the simultaneous testing of many genes for pathogenic and likely pathogenic variants, often at costs comparable to single-gene genetic testing. Hereditary cancer multigene panel testing includes cancer site-specific panels, high- or moderate-risk gene panels, guideline-based panels, and pancancer panels.[17] Given the numerous panel testing options, clinicians must pay special attention to choose the most appropriate panel of genes based on each individual patient's differential diagnosis. For instance, genetic testing for pathogenic variants in both CDH1 and CTNNA1 is recommended when testing criteria for HDGC is met. Additionally, analysis of promoter 1B in the APC gene must be included when testing for GAPPS. For more information about selecting the appropriate genetic test, see the Determining the Test to be Used section in Cancer Genetics Risk Assessment and Counseling. Multigene panel testing implications This section briefly summarizes the implications for patients undergoing multigene panel testing for hereditary gastric cancer syndromes. For more information about multigene panel testing, including genetic education, counseling considerations, and research, see the Multigene (panel) testing section in Cancer Genetics Risk Assessment and Counseling. Multigene panel testing can present challenges, including the unexpected discovery of pathogenic variants in genes that would not have been considered based on the patient's personal and/or family history. Pathogenic variants in many hereditary cancer genes can increase an individual's risk of developing more than one type of cancer. For instance, CDH1 pathogenic variants are associated with increased risk for both diffuse gastric cancer and lobular breast cancer.[14,18,19] Gastric cancer risk management dilemmas arise when individuals with only a personal and/or family history of breast cancer find an unexpected CDH1 pathogenic variant on multigene panel testing. This test result can complicate medical decision-making, since these individuals would not normally participate in enhanced gastric cancer screening based on their personal or family histories alone. Furthermore, there are no effective gastric cancer screening recommendations for CDH1 carriers. Therefore, all individuals with a pathogenic variant in CDH1 are counseled about the option of risk-reducing total gastrectomy, a surgery with postoperative complication rates as high as 10% and a high likelihood of chronic sequelae.[12,20] The discovery of an unexpected CDH1 variant on multigene panel testing in the absence of a family history of gastric cancer is a challenging situation that is increasingly encountered in clinical practice.[21,22] Pretest genetic counseling is recommended when patients undergo multigene panel testing. Pretest genetic counseling may include a discussion about potential unexpected genetic test findings, and the implications of identifying pathogenic variants in genes that are not well understood, including a review of any limitations associated with cancer risk management recommendations.[22,23] Cascade genetic testing Cascade genetic testing identifies relatives who are at risk for a genetic condition by following the logical path of a pathogenic variant in a family. Cascade genetic testing is routinely offered to at-risk relatives once a pathogenic variant is identified. For more information, see the Cascade Genetic Testing of Family Members section in Cancer Genetics Risk Assessment and Counseling. In some cases, the patient will already have advanced gastric cancer when a CDH1 pathogenic variant is found. Many patients do not have symptoms of gastric cancer until it has advanced to later stages. Gastric cancer is often incurable, and only 31% of patients diagnosed with gastric cancer survive 5 years or more.[15] Patients may be declining when they receive their genetic test results and may be unable to communicate this result to family members. Ideally, probands and clinicians work together to identify at-risk relatives for cascade testing.[24] Since gastric cancer screening is not performed in the general population, cascade genetic testing is critical to identify asymptomatic high-risk individuals who may benefit from personalized gastric cancer management recommendations. References: The major heritable syndromes associated with increased gastric cancer risk are listed in Table 1, along with their corresponding susceptibility genes. For more information on CDH1- and CTNNA1-associated hereditary gastric cancers, see Hereditary Diffuse Gastric Cancer. The gastric cancer risk of the other syndromes in this table are described throughout this summary, and hyperlinks have also been provided to other PDQ summaries with additional information. References: Preliminary evidence has linked gastric cancer to other genes that are not typically associated with this type of cancer, such as ATM, BRCA1, BRCA2, BRIP1, PALB2, and RAD51D. Studies suggest a possible association between gastric cancer risk and pathogenic variants in both established gastric cancer genes and in moderate- and high-risk genes that are not typically associated with gastric cancer. However, clinical practice changes are not indicated based on this preliminary evidence. Several studies searched for candidate genes in hereditary gastric cancer cases that were not associated with CDH1 pathogenic variants. Some of these cases may be explained by pathogenic variants in homologous recombination (HR) DNA repair genes, particularly PALB2: In summary, these findings suggest that defects in HR genes increase risk for gastric cancer and highlight a role for the known cancer predisposition gene, PALB2 as a potential candidate gene for hereditary gastric cancer. However, case-control studies are needed to confirm whether PALB2 is associated with hereditary gastric cancer susceptibility. Gastric cancer screening is not indicated for PALB2 carriers. Additional studies investigated potential candidate genes for gastric cancer: Additional studies are warranted to determine if there is a causal relationship between these genes and gastric cancer risk. However, the rate of pathogenic variants found in these studies supports the use of multigene panels in patients with gastric cancer undergoing germline genetic testing. For more information, see the Other Cancer Risks section in BRCA1 and BRCA2: Cancer Risks and Management. References: The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above. Editorial changes were made to this summary. This summary is written and maintained by the PDQ Cancer Genetics Editorial Board, which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® Cancer Information for Health Professionals pages. Purpose of This Summary This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the genetics of gastric cancer. It is intended as a resource to inform and assist clinicians in the care of their patients. It does not provide formal guidelines or recommendations for making health care decisions. Reviewers and Updates This summary is reviewed regularly and updated as necessary by the PDQ Cancer Genetics Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH). Board members review recently published articles each month to determine whether an article should: Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary. The lead reviewers for Genetics of Gastric Cancer are: Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's Email Us. Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries. Levels of Evidence Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Cancer Genetics Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations. Permission to Use This Summary PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as "NCI's PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary]." The preferred citation for this PDQ summary is: PDQ® Cancer Genetics Editorial Board. PDQ Genetics of Gastric Cancer. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/stomach/hp/gastric-genetics-pdq. Accessed <MM/DD/YYYY>. [PMID: 37669413] Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online, a collection of over 2,000 scientific images. Disclaimer The information in these summaries should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page. Contact Us More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website's Email Us. Last Revised: 2025-01-03 This information does not replace the advice of a doctor. Ignite Healthwise, LLC disclaims any warranty or liability for your use of this information. Your use of this information means that you agree to the Terms of Use and Privacy Policy. Learn how we develop our content. Healthwise, Healthwise for every health decision, and the Healthwise logo are trademarks of Ignite Healthwise, LLC.Genetics of Gastric Cancer (PDQ®): Genetics - Health Professional Information [NCI]
Introduction
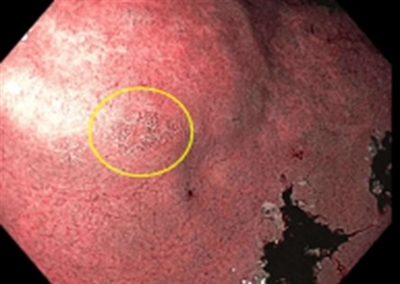
Endoscopic image showing a small area of intestinal metaplasia (circled in yellow) in the antrum of the stomach.Genetic Evaluation in Individuals With Personal and / or Family Histories of Gastric Cancer
Major Genes and Genetic Syndromes Associated With Gastric Cancer
Syndromea Gene Gastric Cancer Risk Gastric Cancer Pathology Gastric CancerSurveillance Other Characteristics CNS = central nervous system; CRC = colorectal cancer; EGD = esophagogastroduodenoscopy; GI = gastrointestinal; GIST = gastrointestinal stromal tumor. a All conditions are inherited in anautosomal dominantfashion, unless otherwise specified. b High-risk lesions include tubular adenomas, polyps with high-grade dysplasia, and pyloric gland adenomas. c Prophylactic total gastrectomy is not recommended prior to age 40 y. However, it may be considered for certain patients, especially those with a family histories of gastric cancer prior to age 25 y. Hereditary diffuse gastric cancer CDH1andCTNNA1 33%–83%[1,2,3,4,5] Diffuse type, signet ring cell carcinoma EGD with multiple random biopsies of the stomach every 6–12 moor risk-reducing total gastrectomy between ages 18–40 yc[6] For CDH1 only: Lobular breast cancer, cleft lip or cleft palate. It is currently unknown whetherCTNNA1is associated with lobular breast cancer or cleft lip or cleft palate Familial adenomatous polyposis syndrome(FAP) APC 1.3%–5% in FAP and AFAP.[7,8] Intestinal type EGD for duodenal surveillance is also adequate surveillance for gastric cancers. Gastrectomy can be considered for patients with high-risk lesions that cannot be managed endoscopicallyb[9] Gastric polyps (fundic gland polyps and adenomas), CRC, colorectal polyps (adenomas), desmoid tumors, duodenal tumors and cancer, thyroid cancer, brain cancer, ampullary cancer, pancreatic cancer, hepatoblastoma Attenuated familial adenomatous polyposis(AFAP) APC Gastric adenocarcinoma and proximal polyposis of the stomach(GAPPS) APC(pathogenic variants/single nucleotide variantin the promoter 1B region ofAPCare associated with GAPPS-7) In GAPPS, gastric cancer risk is significantly increased, but exact risk numbers are unknown[10] Lynch syndrome(also known as hereditary nonpolyposis colorectal cancer [HNPCC]) MLH1,MSH2,MSH6,PMS2,EPCAM 0.2%–9% Intestinal type Upper GI surveillance with EGD (preferably performed in conjunction with colonoscopy) starting at age 30–40 y (or earlier based on the patient's family history). Repeat EGD every 2–4 y[9] CRC, endometrial cancer, ovarian cancer, breast cancer, pancreatic cancer, bladder cancer, biliary tract cancer, urothelial cancer, small bowel cancer, prostate cancer, brain/CNS tumor Peutz-Jeghers syndrome STK11(also known asLKB1) 29%[9] Intestinal type (arising from dysplasia in hamartomas) EGD every 2–3 y beginning in the late adolescence[9] Hamartomatous GI polyps; breast cancer; CRC; small bowel cancer; pancreatic cancer; Sertoli cell tumors of the ovary and testes; cervical adenoma malignum; endometrial cancer; lung cancer; mucocutaneous hyperpigmentation of the mouth, lips, nose, eyes, genitalia and/or fingers Juvenile polyposis syndrome SMAD4,BMPR1A 21% if multiple polyps are present Intestinal type (arising from dysplasia in juvenile polyps/hamartomas) EGD every 2–3 y. If polyps are found, conduct EGD at shorter intervals based on polyp size, number, and pathology[9] Juvenile/hamartomatous colorectal polyps, CRC, hereditary hemorrhagic telangiectasia Li-Fraumeni syndrome TP53 2.8% Possibly intestinal type Breast cancer, adrenocortical carcinoma, sarcoma, brain/CNS tumor, leukemia Other Genes Associated With Gastric Cancer
Latest Updates to This Summary (01 / 03 / 2025)
About This PDQ Summary
Our Health Library information does not replace the advice of a doctor. Please be advised that this information is made available to assist our patients to learn more about their health. Our providers may not see and/or treat all topics found herein.Genetics of Gastric Cancer (PDQ®): Genetics - Health Professional Information [NCI]



